So, how do we contain the hydrogen?
 Producing the hydrogen is not enough. Safe storage methods have to be implemented before a car can operate.
Here, we will describe different storage techniques.
Producing the hydrogen is not enough. Safe storage methods have to be implemented before a car can operate.
Here, we will describe different storage techniques.
At normal temperatures and pressures hydrogen is in a gaseous state, whereas gasoline is a liquid. When contained in the same volume there will be more molecules of gasoline present then that of hydrogen. This means that the energy per unit volume of gasoline is much larger, presenting a serious problem [9]. To overcome this roadblock, hydrogen can be stored in liquid form in the automobile by placing it under an immense pressure of 1000 atm or cooling it to 20 K [10]. These conditions require a great deal of energy and heavy insulation raising questions of safety.
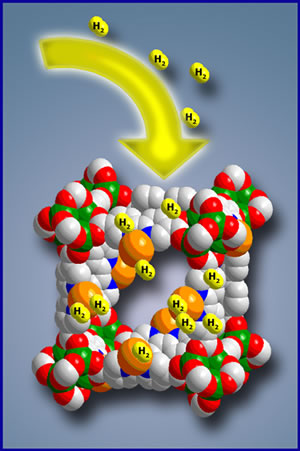
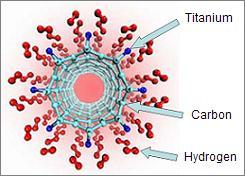
A better way to store hydrogen is to absorb it into the 'pores' of solids such as metal hydrides and carbon nanotubes. All solids have a specific arrangement of atoms called a lattice structure. In between the atoms are spaces where the hydrogen gas molecules can be trapped [11]. Solids absorb hydrogen naturally at slow rates, however, this rate can be increased by placing the solid under pressure. To speed the desorption process, the material can be heated 12]. Research is now being done into using carbon nanotubes to store hydrogen because metal hydrides are often heavy and expensive.
 |
 |
Metal hydride method [13]. |
Carbon nanotube method [14]. |
After reading about fuel cells and hydrogen, do you think you have a better understanding of it all now ? Try your luck here.