Obtaining H2 gas is not as easy as you think!
 Most reformation is done at oil refineries such as this one [6]. | In this section, we will see how hydrogen is produced. Mainly, we will look at thermal reformation. Also, a less in depth explanation of electrolysis will be presented. Hydrogen is the most abundant element on earth, mostly as part of compounds such as water and hydrocarbons. The extraction of hydrogen from these compounds and its delivery to the automobiles maybe the biggest obstacle facing the implementation of fuel cells into the economy. The production of hydrogen has to be efficient and environmentally "clean", otherwise the high cost of fuel cell cars would not be worth the effort. In the USA, 95 % of hydrogen is obtained through a process called thermal reformation of methane [4]. |
In the process, methane reacts with steam at high temperature (700 - 10000oC) and pressure (3-25 bar) to produce hydrogen (H2) and carbon monoxide (CO) [4]. This reaction is endothermic and requires an energy of 201 kJ/mol. A secondary reaction occurs when carbon monoxide reacts with water to produce carbon dioxide and small amounts of heat. The second reaction is exothermic, and releases 31 kJ/mol [5].
| If the CO is not filtered out properly from the H2 it can damage the thin platinum layer in the fuel cell membrane. Although steam reformation produces some CO2, when compared to internal combustion engines it reduces the CO2 emissions by 60% [4]. Among the other processes used for producing hydrogen, the most useful is the electrolytic process, where water is split into hydrogen and oxygen. This is similar to the workings of a fuel cell. At the anode the water is split into hydrogen ions and oxygen. The extra electrons travel through an external circuit to the cathode. The ions also travel through a membrane to the cathode, where they meet up with the electrons to combine into H2 molecules [8]. This method is the cleanest way for producing hydrogen, since it results in nearly zero greenhouse gasses. |
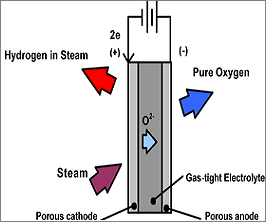 The process of electrolysis [7]. |
Now that we have talked about hydrogen production, there is one more topic to discuss. To learn how hydrogen is stored click here.