What in the world is a fuel cell?
 In this section, we will concentrate on the structure of a fuel cell and the chemical equations taking place within.
In this section, we will concentrate on the structure of a fuel cell and the chemical equations taking place within.
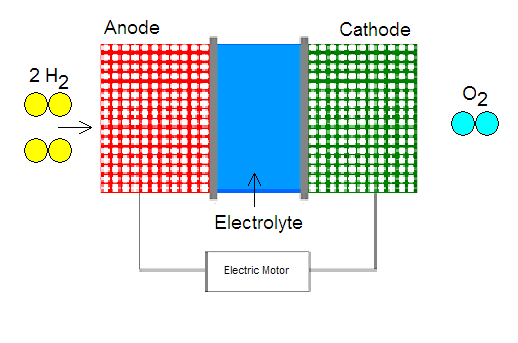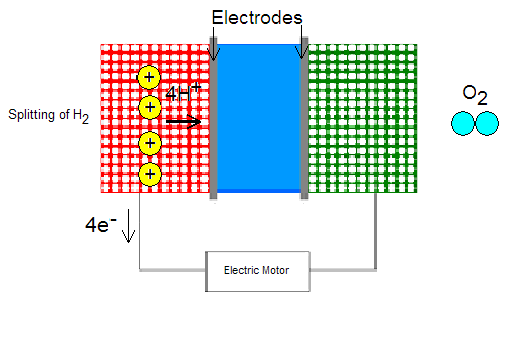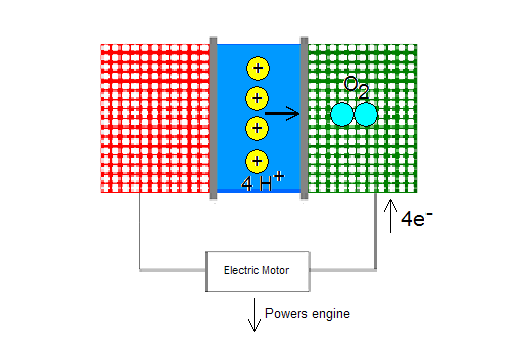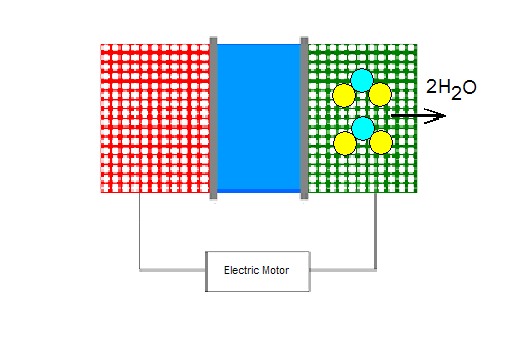
A fuel cell is a device that is fairly similar in structure to a common battery. Its purpose is to create electrical energy using hydrogen gas. This process starts at the anode side of the fuel cell where hydrogen is ionized to produce a proton and an electron. The chemical reaction for the ionization of the hydrogen is [2]:
2H2 -> 4e- + 4H+
The electrons travel through an external circuit to the cathode, thus creating electricity to power the automobile. The protons, on the other hand, go to the cathode through the middle part of the cell called the electrolyte. It is made of a material that allows only one-way traffic of protons, from the anode to the cathode. The fuel cell can be constructed in different ways, but the primary candidate for hydrogen powered cars is the proton exchange membrane (PEM), which includes a thin layer of platinum. This metal acts as a catalyst for the ionization of hydrogen gas. The main drawbacks of such a membrane are the high cost of platinum and its intolerance to carbon monoxide (CO), which destroys platinum's ability to work as a catalyst [2].
When electrons and protons “arrive” at the cathode, they participate in a reaction with oxygen to produce water (H2O).
4e- + 4H++O2 -> 2H2O
A single fuel cell gives approximately 0.7 volts of power, which is not nearly enough to propel a car. This problem is easily fixed by combining many fuel cells into a "stack" [2]. To produce enough energy for a car, about 400 individual fuel cell have to be welded together [3]. Thus, they create a radically new, clean and more efficient way to power a vehicle.
This concludes the section about fuel cell mechanics, but don't worry, there's more to come. To check out how hydrogen is produced click here.