How is Ethanol Produced?
 In these sections we examine how the same starch that we eat to power our bodies
can be harnessed to fuel an automobile for a road trip. We also take a look at
some of the benefits and drawbacks of ethanol.
In these sections we examine how the same starch that we eat to power our bodies
can be harnessed to fuel an automobile for a road trip. We also take a look at
some of the benefits and drawbacks of ethanol.
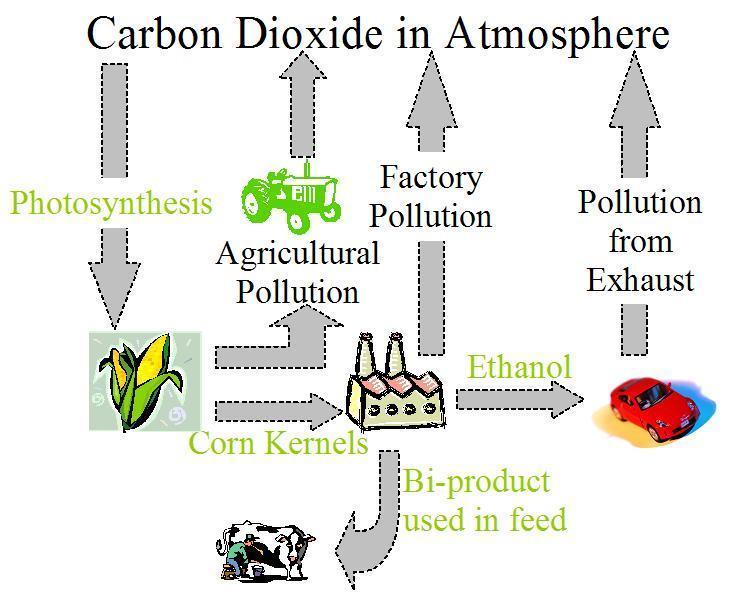
Ethanol production is little more complicated than one might think, as there are a variety of methods and crops that may be used to produce ethanol. Standard ethanol is produced from the starches or sugars contained in the seeds of different crops, but cannot be manufactured from the sugars found in animals. Differing climates mean that nations will use different crops to produce ethanol, for example while in Brazil and India sugarcane is the primary crop used in ethanol production, in Canada and the USA corn is predominantly used [3]. Ultimately though, regardless of the crop used, the basic process is similar:
|
1) The crop is grown, harvested and transported to the ethanol facility [4]. |
|
There are two primary methods for separating the starch from corn, called dry mill and wet mill. In dry mill, the seed is ground into a flour before being mixed with water. In wet mill, the seed is allowed to soak in dilute sulfurous acid, before being ground down further. A diagram showing the simplified version of the cycle used to produce ethanol from corn is shown above [4].
How Much Energy Can We Get From Ethanol?
There are many significant issues concerning ethanol production, its overall energy efficiency and whether it is truly environmentally friendly. Some scientists have argued that the whole overall process actually requires more energy than is obtained in an ethanol fuel, however the majority of studies have shown the opposite [5]. For example, Dr. Wang from the Argonne National Laboratories has estimated that for every 1kJ of ethanol fuel energy produced from corn it only requires 0.74kJ of energy from fossil fuels. Compared to gasoline, where 1.23kJ of energy is required to make 1kJ of fuel energy available on the market [6].
|
For the sake of argument, lets do a quick calculation ourselves using values provided by the Argonne National Laboratories. They estimate that for each litre of ethanol that 21200kJ of usable energy can be provided, while fossils fuels must provide 7440kJ of energy to produce the corn and another 12350kJ in order to refine the corn into ethanol [6]. In total then, while we must input 19790kJ of energy from fossil fuels, we obtain 21200kJ of usable energy from ethanol. |
|
|
See the Energy Comparison section for more comparisons between ethanol, hydrogen, gasoline and natural gas.
What are Ethanol's Drawbacks?
Depending on the crop used and the location it is grown there are other environmental aspects that must be considered. For instance, in Brazil the rapidly expanding demand for sugarcane production for ethanol has to be balanced with the need to preserve the rainforest and other habitats. In Canada and the USA, where corn is used, soil degradation and other forms of pollution from corn production must be considered. As pointed out by Dr. Pimentel in a 2003 study, corn production is a major cause for soil erosion, and requires large amounts of nitrogen fertilizer, which contributes to water pollution [7]. In addition to this, ethanol leakage poses a serious threat to the groundwater supplies. Ethanol's corrosivity increases the likelihood of leakage from storage, and ethanol itself is more likely to pass through table rock into groundwater. Once in the water, ethanol exacerbates the problem by increasing the ability of benzene to dissolve in water [8].
Beyond the environmental concerns even if all the corn currently produced in the USA was converted to ethanol it has been estimated that it would only satisfy 12% of the current demand for gasoline [3]. So despite the great potential that ethanol has, it seems like that we have reached a dead end. Or have we?
Where can we go from here with ethanol? Lets find out.