 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|
Ramifications to physics |
|
Einstein's idea of a light particle wasn't accepted immediately by everyone. Maxwell's wave theory of light was well established and had strong mathematics to support it. Einstein propsed the idea that light was composed of paricles and was extended by de Broglie to matter, in particular electrons, that appeared to exibit wave properties as well. With Einstein's application of quantized energy also came the first confirmed quantum model. Quantum mechanics is the study of physics on very small (atomic) scales. Bohr constructed a model of a hyrdrogen atom, where the electron could only exist in quantized energy levels. His model was later confirmed with atomic spectra and this was the first solid evidence of quantum theory. 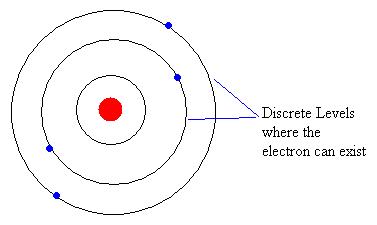 As we approach the speed limitations of traditional electronic systems used in computing, physicists are working in the field of quantum computing, where the quantum states of an atom are used to perform mathematical operations. |
