Process
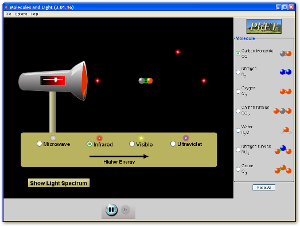
If we boil down what light is to its root, we can think of light as travelling bundles of energy. Depending on how we measure it, light will act as either a wave or a particle (photon); both of which are important in its interaction with matter. Click on the subtitle above to visit an introductory website to review some basic aspects of light and the different regions of the electromagnetic (EM) spectrum. It will show you the various types of radiation, their properties, and some of their applications. Be careful, visible light as we know it is only a small part of this spectrum and is part of what we call EM radiation. Be sure to keep track of the different types of radiation and the differences between them. This is summarized in the picture below:
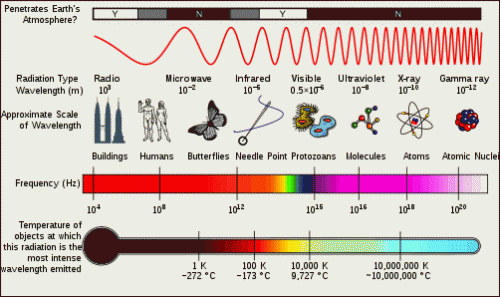
The applet below relates the frequency, energy, and wavelength of each region within the EM spectrum. Pay special attention to the wavelengths of each region and their relation with energy / frequency. Note how the EM spectrum is continuous (by dragging the wavelength selector across the screen) and one form of radiation gradually merges into the next.
Now that you have an understanding of what EM radiation is, click on the subtitle, "Radiation and Matter" to learn about what happens when an EM wave hits a molecule or atom. Basically this boils down to one of 3 processes: absorption, scattering (light absorbed and re-emitted at a random angle), and reflection. Which process will occur is somewhat random (notice how the intensity [ or amount of photons of light] changes even though the laser beam stays the same?).
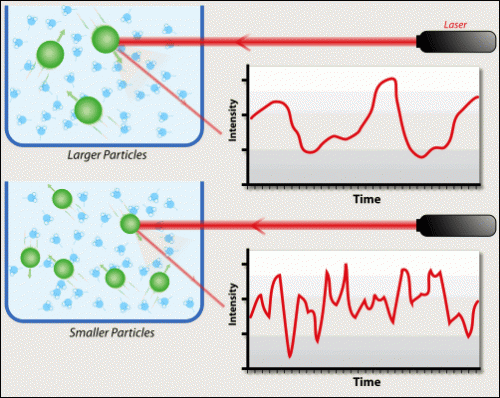
"Somewhat random" what could we mean be this? It is true that there's a chance for each process to happen, however one will generally be favoured depending on several factors that make it the most probable. You are going to discover what some of these factors are by playing with the applet below. What do you notice about the amount of light that is absorbed and reflected as you change the type of light? Does this depend on the molecule you're using? Why do you think this is?
PhET Interactive Simulations
University of Colorado
http://phet.colorado.edu.
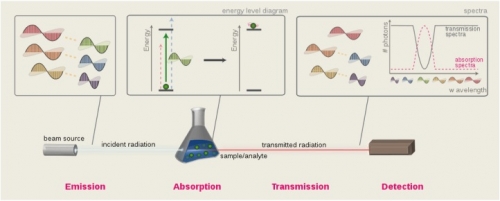
Biosensors measure the changes in how a molecule reacts to light due to its surrounding environment. If the molecule can bind to other materials, these changes can be used to signal the presence of the material. These "materials" can range from glucose, to DNA, to toxic chemicals. The picture below is an overview of the ideas behind this process. A beam of light (first box) passes through a sample. As this happens, some wavelengths are absorbed more than others (here we see the green wavelength has been absorbed in the second box). What's left of the beam of light (called the transmitted beam) is measured by the detector and the amount of photons at each wavelength creates a transmission spectrum. If we measure the missing photons instead, we get an absorption spectrum.
Watch the video "Synergy: Biosensors with Yi" Lu by clicking on the "Basics of Biosensors" link. This video shows how we can detect toxic chemicals using biosensors. The biosensor shown in this video works by measuring changes in the number of photons (intensity) at a given wavelength and colour (the aborption spectrum has is shifted leading to a change in colour). The term fluorescence just means something is absorbing a photon of one wavelength and emitting a photon at a different wavelength; it "glows."
Now it is time to learn about another application of light in a different type of sensor. Click on the "Cancer Detecting Biosensors" link and watch the video "Gold Nanoparticles and Cancer Detection" shows how tiny particles of gold (nanoparticles) are used to see cancer cells that normally wouldn't be seen in a tissue. Here the nanoparticles bind to the cancer cells and increase the amount of light reflected from the cells. As these particles cannot bind to normal cells, the cancer cells appear brighter and can easily be seen under a microscope.
Now you have a fundamental understanding of how light interacts with matter and how we can exploit these interactions. The assignment given to you by your teacher will require you to apply the knowledge learned in this WebQuest in new and interesting ways.