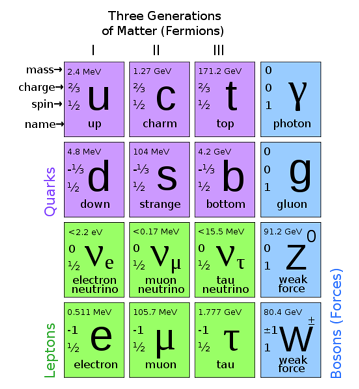
The different quarks are usually referred to as different "flavours", and they are the up,
down, strange, charm, top and bottom quarks. The different flavours are usually separated
into three groups which are sometimes referred to as the first, second and third generation
particles.
The second and third generations are highly unstable and decay quickly into first generation particles. All the flavours of quarks have an electric charge of either (2/3)e or -(1/3)e, where e is the charge of an electron (1.602 x10-19C). You may recall from chemistry that electrons have a characteristic about them called "spin" or a "spin quantum number". This property can be considered in all particles and each particle has its own spin quantum number. Similar to the electron, all the quarks have a spin number of 1/2. An odd property known to quarks and gluons only is a so called 'colour' charge. Colour charge doesn't refer to a visual colour. Moreover, this property is another quantum number, similar to the spin quantum number.
In order to understand why we need colour charge we need to take a quick break to look at the "Pauli Exclusion Principle". The Pauli Exclusion Principle states that no two particles can be in the same space area if they share the same quantum numbers.
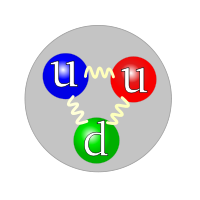
Getting back to why we need colour charge. If you recall the proton being made of three quarks, those three quarks were one down quark and two up. To satisfy the Pauli Exclusion Principle the colour charge quantum number was added since the three quarks all have the same spin number and are in the same area.
Quarks can take one of three colours, red, green, or blue. The colour charge is only taken into account in quantum mechanics when dealing with distances that are smaller than the atomic nucleus.
| Quark | Symbol | Generation | Electric Charge (e) | Spin Quantum Number |
|---|---|---|---|---|
| Up | u | I | 2/3 | 1/2 |
| Down | d | I | -1/3 | 1/2 |
| Charmed | c | II | 2/3 | 1/2 |
| Strange | s | II | -1/3 | 1/2 |
| Top | t | III | 2/3 | 1/2 |
| Bottom | b | III | -1/3 | 1/2 |