 THE TWO SCHOOLS OF THOUGHT
THE TWO SCHOOLS OF THOUGHT

The phenomenon of Brownian motion caused much debate on the possible causes for this motion. These debates lead to two opposed and contradicting sides. There were the ENERGETICS, lead by William Ostwald, and the ATOMISTS, lead by Ludwig Boltzmann.
 THE GREAT ATOM DEBATE
THE GREAT ATOM DEBATE


| ENERGETICS | ATOMISTS | |
|---|---|---|
|
1. View the world through macroscopic properties 2. Use Classical Thermodynamics to explain phenomenon 3. NO such thing as pariticles! |
1. View the world through microscopic properties 2. Use the Molecular Kinetic Theory of Heat to explain phenomenon 3. Fluids behave AS IF they are composed of particles |
Now let's take some time to discuss what each of these points mean.
Point 1:
The Energetics believed that everything in the world could be explained by using macroscopic properties such as energy, volume, pressure, and temperature.
The Atomists viewed the world by modeling gases and fluids as though they were composed of tiny particles that moved around, this lead them to having to use statistics to explain phenomenon.
Point 2:
The Energetics used the theoretical framework of Classical Thermodynamics 12 to describe phenomenon mathematically. The most basic equation from this formulism is given by the ideal gas law, which states, as before, that PV = CT.
The Atomists used the theoretical framework of the Molecular Kinetic Theory of Heat 12 to describe phenomenon mathematically. The most basic equation from this point of view relates the kinetic energy, or the moving energy, of a single particle to the temperature by a constant known as Boltzmann's Constant. This may be expressed as
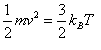 . In this equation
m is the mass of the particle, v is its velocity, k is Boltzmann's constant and T is the temperature.
. In this equation
m is the mass of the particle, v is its velocity, k is Boltzmann's constant and T is the temperature.
Point 3 The Energetics did not believe that matter was discrete. They believed that you could cut up matter into smaller and smaller pieces indefinitely.
The Atomists on the other hand, viewed matter in much the same way as Democritus, by saying that eventually you will reach a point where you can no longer cut any further.
So far the fight between the Energetics and the Atomists is pretty even until the question of irreversibility.