RAPID TEST FOR PREDICTING ALKALI REACTIVITY: PROMISES AND PROBLEMS
PETER P. HUDEC, Geology Department, University of Windsor Windsor, Ont. N9B 3P4
Hudec, Peter P., 1990, Rapid tests for predicting alkali reactivity: Promises and problems, in: Canadian developments in testing concrete aggregates for alkali-aggregate reactivity, Ministry of Transportation, Ontario, Report EM-92, pp. 111-117.
1. ABSTRACT
Because of inherent slowness of Alkali ‑ Aggregate Reaction (AAR) both in nature and in existing ASTM and CSA tests, new rapid tests have been developed to evaluate the potential of aggregates for AAR. The Oberholster and Davies hot 1N NaOH test appears to be a good, if severe, rapid test. A variation of this test has been used for the last three years in this laboratory, with generally good, but occasionally quirky results.
The AAR potential is measured by expansion. The test was performed on 19mm and 25mm diameter, 70mm length cores of mortar and concrete. The specimen size had little influence on test results. Consistent variability in expansion during the same test was seen in samples cored from the same block when all other conditions were constant. Additionally, variability in the expansion of the samples made from the same aggregate, the same mixture and the same curing condition but at different times was also evident. Small changes in procedure and/or conditions of the mixture resulted in significant changes in expansion. Different AR aggregate exhibited different rates of expansion, some were early rapid, some late rapid expanders. Thus expansion after a given length of exposure (14 days), or rate of expansion may not accurately evaluate the aggregate.
Contrary to expectations, pessimum effect was observed in the hot NaOH environment. An osmotic theory of AR expansion is offered as an explanation for the pessimum phenomenon.
Given all the variables that may affect the test outcome, it is suggested that every rapid test include two standards: non‑reactive and reactive aggregate standard. All AAR expansions should be expressed both in terms of absolute and relative expansion to the two standards. The expansion readings should be adjusted to the mean expansion of the standard. Initial and final rates of expansion should also be used in evaluation.
2. INTRODUCTION
Alkali silica(te) reactivity (ASR) has been recognized as a growing problem in many areas of the world. Since ASR was first recognized in early, more aggregate types have been found to be susceptible. Also, as demand for aggregates rises, new, untested sources are being developed. AR has to be suspected in all materials that have silica or silicate content - even some carbonates with silicate minerals are suspect as alkali-silica reactive.
Alkali silica reaction is slow, but alkali silicate reaction even slower. The 'standard' ASTM and CSA tests that simulate the optimum natural conditions for alkali reactivity are also slow. However, when a new aggregate source is developed, the need for the material is immediate. Six to twelve month wait for test results is not acceptable. In some cases, concrete already placed may be questioned as to its reactivity. Taking large samples is expensive, and may not be possible without affecting the integrity of the structures
A variety of rapid tests have been under development by different researchers during the last several years. Of these, the test proposed by Oberholster and Davies appears to have a good correlation to the 'standard' tests. The rapid AR test described in the paper is a modification of the Oberholster and Davies test. The results obtained outline some advantages and some drawbacks with this test, and with similar accelerated tests in general.
|
|
Figure 1 Schematic diagram of the LVDT length measuring apparatus.
2.1 Details of Testing Method:
The Oberholster test uses mortar bars of the ASTM C227 specification, and 1N NaOH immersion at 80oC to accelerate the alkali reactivity. This procedure was modified for use in this laboratory. Briefly, rather than casting three mortar bars for each test, a single block of either concrete or mortar is cast. After 24-h set, the block is de-moulded and cured for 24h in 80oC water. Three cores of either 19mm or 25mm diameter are cut from the block; the remainder of the block is stored moist for additional testing, if required. The cores are kept moist at all times, and are squared and 'dimpled' at ends to allow them to fit into the measuring equipment.
The equipment (Figure 1) consists of a double LVDT (linear variable differential transformer). One LVDT is used to set the original length of the sample, and the second reads the changes in length during the experiment. The sample length is measured seven times, and the 'cleaned' mean (to +/-1 standard deviation) is extracted. The means for the three cores per sample are likewise 'cleaned'. The final result is considered to be a very accurate measure of the sample's length. The accuracy is necessitated by the small length of the sample -about 70mm.
The cores are placed in the hot 1N NaOH environment for 2 days, cooled to room temperature to facilitate handling, and their length is measured. The cores are then returned to the hot environment; the cycle is repeated for a minimum of 14, and maximum of 21 days. The number or cycles depends on the nature of expansion observed.
2.1 Reactive Aggregates Tested:
Four types of reactive aggregates were used in this study. Three of these were obtained from the stockpile of reactive aggregates maintained by Ministry of Transportation, Ontario. They were alkali-silica carbonate from Spratt quarry near Ottawa, Ont., alkali-silicate crushed gravel from near Sudbury, Ontario, and alkali-carbonate reactive crushed stone from Kingston, Ontario. The fourth, an alkali-silica reactive aggregate was obtained from crushed chert cobbles from Putnam, Ontario (east of London). A non-reactive crushed limestone from research stockpiles at the University of Windsor was used as a control.
3. EXPERIMENTAL RESULTS
The experimental results presented here have been selected from along-term testing project of concrete and mortar containing reactive aggregate. Much of the testing involved treating aggregate, concrete, or mortar with various chemicals to test their effectiveness in controlling AR. In each of the tests an untreated control set is run; the results given here are collected from the control runs.
3.1 Comparison of Concrete and Mortar Expansion
|
|
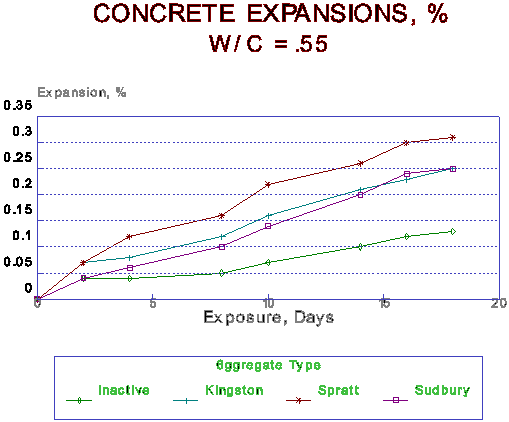
Figure 2 Expansion of Concrete under Hot NaOH conditions.
The typical expansions obtained for concrete specimens containing -9.5mm graded aggregate are given in Figure 2. Similar results are shown in Figure 3 for mortar specimens containing the same aggregate. The figures show a systematic parallelism of the curves which can be ascribed more to instrument drift than to actual changes in the expansion behaviour.
|
Figure 2 Expansion of Concrete under Hot NaOH conditions. |
|
|
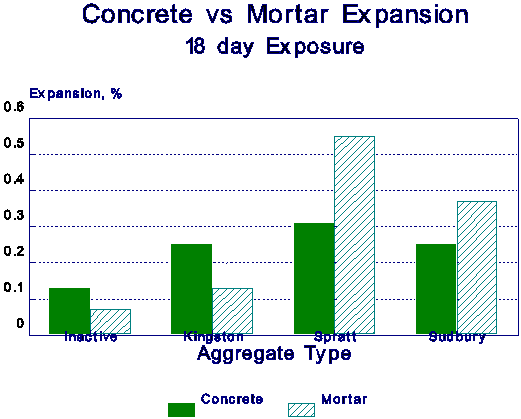
Figure 4 Comparison of concrete and mortar expansions.
When the expansion of the concrete and mortar are compared (Figure 4), it is seen that at 18 day exposure, the mortar expands more in reactive alkali-silica(te) specimens than in does in reactive alkali-carbonate aggregate containing specimens. This type of behaviour was consistently noted in all experiments. Thus, although both concrete and mortar can be used to test for AR, somewhat different results may be expected, depending on the type of aggregate used. This is probably more of a non-equilibrium or rate of reaction problem dictated by the difference in the surface area of the reactive aggregate. The alkali-carbonate reaction involves the entire aggregate, whereas the alkali-silica reaction is more of a surface phenomenon. These results are only preliminary, and need to be investigated further.
3.2 Effect of specimen diameter on the rate of expansion.
|
|
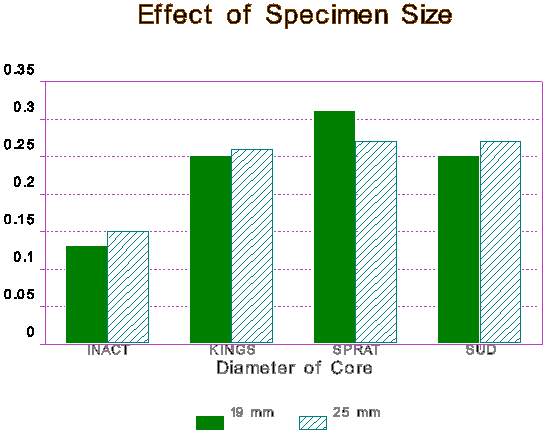
Figure 5 Effect of Specimen size on the Rate of Reaction.
The size (diameter) of specimen did not seem to have much effect on AR expansion. The results on two sizes, 19mm and 25mm diameter cores, are shown graphically in Figure 5. The work was done on concrete specimens cored from the same cast block, and shows expansion after 14 days of exposure. It is expected that similar outcome will be obtained on mortar specimens. This work suggests that smaller cores can be obtained from existing structures for rapid testing for AR, and the results will be comparable. Again, the data is preliminary, and more work needs to be done on a greater variety of sizes.
3.3 The Pessimum Effect.
The current understanding of the Pessimum Effect in AR holds that the AR expansion is controlled by both the amount of alkalies and the amount of reactive aggregate in the concrete - the alkali/reactive silica ratio. For a given amount of alkali, if the amount of reactive aggregate is increased beyond certain level, the expansion falls off. If the amount of alkali is increased, the expansion will increase as the amount of reactive aggregate increases.
Thus, in rapid AR testing with abundant alkali in the form of hot NaOH solution, the convention would dictate that as the amount of reactive aggregate is increased, the expansion will also increase. This proved not to be the case, as is shown in Fig. 6.
|
|
Figure 6 The effect of increasing reactive chert content in mortar specimen.
The reasons for the pessimum effect need to be reconsidered. There is an overabundance of alkali in the accelerated hot NaOH environment, so the ratio of alkali/silica does not change significantly as the proportion of reactive silica is increased. According to currently held ideas, the expansion should continue, but perhaps at somewhat lower rate as the reactive silica proportion is increased. However, as Figure 6 shows, it does not. The explanation probably lies in the causes of expansion due to AR.
The AR expansion is produced by the alkali-rich gel imbibing low alkali water by osmosis. The amount of gel produced is indeed a function of the available reactive silica and the alkalies. However, the distribution and distance between the reactive silica particles within the mortar or concrete for a given content of alkalies is important. Several investigators have shown that by maintaining the alkali/reactive silica ratio constant while reducing the particle size, the expansion of the specimen. Reducing the particle size increases the number of particles of reactive material in the mix, and decreases the distance between them.
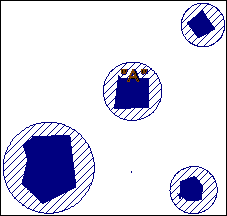
If each particle produces silica gel, and if sufficient alkali is present to make that silica gel imbibe water, the particle can be considered as an expansive centre, deriving its water from the surrounding paste. Each particle can be considered to have an osmotic sphere of influence, as shown in the cartoon of Figure 7. As long as a high osmotic potential exists, particle will continue to produce expansive stress. If, however, there are sufficient number of particles present so that their spheres of influence overlap or interfere with each other, the osmotic differential will be decreased, and the water flow will be reduced or cease, as illustrated in the cartoon of Figure 8.
|
Figure 7 AR aggregate concentration at pessimum or less: water is free to migrate to high potential osmotic zones surrounding each particle. |
|
Figure 8 AR aggregate concentration at greater than pessimum: overlapping high osmotic potential zones reduce osmotic gradient around each particle. |
The above theory also explains the beneficial action of pozzolans in controlling expansion due to AR. The fine particles of the pozzolanic material can be thought of as individual centres around which an osmotic potential zone is set up. If the particles are in sufficient concentration so that the osmotic zones overlap, the osmotic difference within the concrete diminishes, and the expansion is prevented.
3.4 The reproducibility of the accelerated testing method.
|
Figure 9 Expansion of Inactive aggregate as measured over 5 runs. |
Water-cement ratio, in so far as it controls the void ratio in the concrete and mortar, was found to significantly affect the expansion rate. W/C ratios of more than 0.55 decreased the AR expansion. The variations in conditions of testing were also found to be important. Not all of these have been identified. In one case, all AR and non-reactive specimens contracted throughout the test.
In this rapid AR test, the specimens are allowed to cool down to room temperature for measurement purposes. This appears to introduce an unknown variable to the procedure. In the next set of tests, the measurements in hot and cold state will be compared.
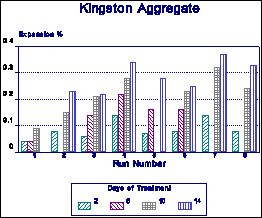
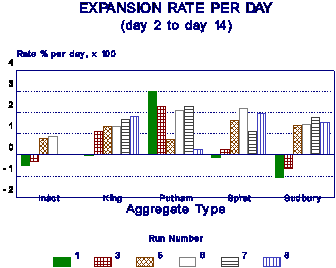
It has also been suggested by various workers that rather than the absolute expansion, the rate of expansion be used as a measure of AR. Figure 12 shows that there is a significant variation in the rate of expansion for different runs. The rate of expansion is very much aggregate-dependent. Some aggregates are slow early expanders (Sudbury), others are rapid early and slow later expanders (Putnam).
|
Figure 10 Repeatability tests on Kingston (ACR) aggregate. |
|
Figure 11 Repeatability tests on Sudbury (ASR) aggregate. |
|
Figure 12 Rate of expansion as measure of AR susceptibility. |
The variation in the results obtained above would suggest that the rapid method as described here is not reliable. However, by adjusting the 'raw' data to that obtained from the 'inactive' standard, the results come to acceptably comparable levels. This emphasizes the need to run standards with each test. It is suggested that both inactive, and known AR active samples be measured with the unknown samples being tested. In this way, the variations in expansion due to specimen preparation, conditions of the test, and the testing method can be equalized.
The method of curing the specimen also has some influence on the results. Figure 13 shows the difference in expansion between the 'standard' 28-day curing of specimens containing ASR Putnam aggregate and the same mixture cured at 80oC and 40oC in water. As can be seen, the 28-day cure shows marginally greater expansion than does do the rapid, one day cure methods at elevated temperatures. Rapid curing is therefore an acceptable way of speeding up the rapid test. Curing at 80oC has the added advantage of using the same oven settings for both curing and expansion testing.
|
Figure 13 Effect of rapid vs 'standard' curing methods on AR expansion in rapid tests. |
4. CONCLUSIONS
The results presented above would suggest that rapid testing for Alkali Reactivity is viable, providing that considerable care is taken in minimizing variability of both sample preparation and conditions of testing. The following summarizes the findings:
1. Both concrete and mortar specimens can be used. Mortar gives somewhat greater expansion than does concrete.
2. Specimen size is not a factor in the rapid AR testing.
3. Void content of the specimen is one of the main controls of expansivity. Thus, the water/cement ratio, the physical process of mixing of the wet mortar or concrete, rodding or vibrating during casting (in so far as these affect the void ratio) must be strictly controlled.
4. Pessimum effect, although not expected in rapid NaOH-based AR testing, was found to be present. It is suggested that the distribution of the reactive particles in the mixture and the inter-particle distance between them rather than the alkali/reactive silica content govern the amount of expansion.
5. Aggregates of know response to AR testing should be used as part of each testing run, and the unknown aggregate material compared to them. Both inactive and active aggregates are suggested. Relative expansion to the standards rather than absolute numerical limits should be used to judge the unknown material.
6. The rapid AR testing method suggested here allows the use of relatively small specimens in the form of cores, cut from either the cast test block or from existing concrete structure. The remainder of the block can be used for either repeat tests or for other testing procedures.
5. REFERENCES
1. Stanton, T.E., The Expansion of Concrete Through Reaction Between Cement and Aggregate, Proc. Am. Soc. Civ. Engrs, 66, 1781-1811, 1940.
2. Oberholster, R. E., and Davies, G., An Accelerated Method for Testing the Potential Alkali Reactivity of Siliceous Aggregates, Cement and Concrete Research, 16, 181-189, 1986.
3. Hudec, P.P., and Larbi, J.A., A study of Alkali-Aggregate Reaction in Concrete: Measurement and Prevention, Part I: Measurement - Development and Prevention, Cement and Concrete Research, 19, 905-912, 1989.
4. Hobbs, D.W., Alkali Silica Reaction in Concrete, Thomas Telford, 22-25, 1988.
5. Hobbs, D.W. and Gutteridge, W.A., Particle Size of Aggregate and its Influence upon Expansion Caused by the Alkali-Silica Reaction. Mag. Concr. Res., 31, 235-242, 1979.
6. Diamond, S. and Thaulow, N., A Study of Expansion Due to Alkali-Silica Reaction as Conditions by the Grain Size of the Reactive Aggregate. Cement and Concrete Research,, 4, 591-607, 1974.