EVALUATION OF VARIOUS CONCRETE DEICERS
P.P. HUDEC*, C. MACINNIS**, F. ACHAMPONG**
*Department of Geology, **Department of Civil and Environmental Engineering
University of Windsor, Windsor, Ont. Canada
Abstract
Rock salt (NaCl) remains in use as the principal road deicer in spite of its well‑know corrosive properties. It has a eutectic of ‑23oC, good ice melting rates at low temperatures, and above all, is relatively inexpensive. In the last several years, a search has been conducted for a non‑corrosive deicer to replace salt; a leading candidate has been calcium magnesium acetate (CMA), which currently costs about 10 times more than NaCl. In addition to the cost disadvantage, CMA is effective to only about ‑5oC. Two types of alternatives exist to NaCl: An additive to counteract its corrosiveness without significantly affecting its deicer properties, or a non‑corrosive, cost and performance effective replacement. Both alternatives have been investigated in these studies. Phosphate salts have long been suggested as alternate deicers. However, phosphate salts by themselves are not effective, though they do provide corrosion protection. Mixtures of NaCl and various phosphate salts have been shown to be effective non‑corrosive deicers. Ca‑ K‑ Na‑, NH‑ phosphates all show some promise, especially the mono‑valent phosphates. The Ca phosphate is insoluble, but when applied in the acidified state, precipitates in concrete pores and continues to offer protection.
Potassium acetate (KAC) has been shown to have superior de‑icing properties, equalling the deicing properties of calcium chloride and far exceeding those of CMA. As with CMA, KAC is non‑corrosive and environmentally friendly.
Results of laboratory tests on deicing properties and surface scaling tests for the various deicer combination studies are presented.
Keywords: Deicer, Salts, Durability, Freeze-Thaw, Beneficiation, Adsorption, Cation, Anion, Admixtures, Calcium magnesium acetate, Sodium chloride, Potassium acetate
Hudec, P.P., MacInnis, C., and Achampong, F., 1992, Evaluation of various concrete deicers, 9th Int. Congress on the Chemistry of Cement, New Delhi, India, November
![]()
1. Introduction
Deicing salts have become a necessity for paved surfaces in temperate climates, both to prevent accidents, and to reduce legal liability due to accidents. Ice control or prevention of ice formation is required on a large scale on roads and highways, and on a smaller scale on driveways and walkways of public institutions and homes. For the former, sodium chloride (NaCl), remains the dominant deicer, due to its abundance and low cost; for the latter, a variety of deicers are used, but most common are sodium and calcium chloride (CaCl2),or mixtures of the two. The main problem with the chlorides is their corrosive effect on the reinforcing steel of structures, scaling of concrete surfaces, and the accelerated deterioration of aggregates in both cement and bituminous concretes. A new deicer, or a method of modifying the current deicers to alleviate their undesirable effects is needed.
This paper reports on the initial results of adding other salts to NaCl to modify its behaviour and still maintain its deicing properties. It also reports on a promising new deicer - potassium acetate (KAC) - that has deicing properties similar to that of calcium chloride without the undesirable side effects.
2. Theories of chloride deicer frost damage
Corrosion of steel by chlorides, both the steel embedded in the concrete (reinforcing bars) and the exposed steel (girders, railings, cars, etc.) is well known. Corrosion of rebars is a chemical process, aided by the physical deterioration of the concrete by the freezing and thawing cycles. The physical deterioration theories will be discussed briefly. The understanding of the physical deterioration process, and the role of the deicing salt (NaCl) in it, is essential before an alternate deicer can be found. The main variables in the frost damage of porous materials are the nature of the pore surface, pore and capillary size, pore and capillary size distribution, and the total internal surface area per unit volume.
2.1 Nature of pore surface
All surfaces, including mineral surfaces, posse a surface charge; the strength of charge is a function of the nature of the surface. In minerals that make up the rock used as natural aggregate, the surfaces can be mineral faces (rarely), cleavage faces, or fracture faces. The latter are the most active, since fracture surface breaks randomly across chemical bonds of the mineral; cleavage faces represent a direction of minimum bonding between elements of the crystal and are less active. Furthermore, if the crystal is 'imperfect', i.e., has an incomplete lattice, or a lattice in which under-or over-size elements substitute, or has a lattice that has been tectonically strained, the surfaces of that mineral will possess a greater charge.
The charge on the surfaces is generally negative. The negative surfaces attract polar water molecules and the cations of deicing salts. For instance, the Na+ of NaCl is adsorbed to the surface. Polar water molecules, in turn, are attracted to the cation, increasing the amount of tightly held water above that which the surface would normally hold. The action is similar to that of sorption of cations onto clay particles - the "double layer" theory. The concentration of cations is greatest near the surface, and diminishes gradually away from the surface. Adsorbed water, having lost some of its free energy as heat of adsorption, has lower vapour pressure than 'normal' or bulk water.
2.2 Pore Size
The size of the pore determines the proportion of the adsorbed vs. 'normal' water held in the pore. The water adsorbed at close to 100 percent relative humidity at 20oC completely fills capillaries of up to 5 um radius, as shown by the Kelvin equation. If the material contains a greater proportion of small size pores in the 'critical' range, more will be filled with adsorbed water and the ratio of adsorbed to absorbed water in the porous material increases. The adsorbed water in the pores has lower vapour pressure; the vapour pressure of the water is proportional to the pore radius - thus, the water in very small pores has a lower vapour pressure than water in larger pores. If the porous material is fully saturated by immersion, the larger pores contain mostly bulk, normal vapour pressure water, and a vapour pressure potential exists between large and small pores. Because of this difference, higher vapour pressure water is compelled to move towards lower vapour pressure water by osmosis. The pressure in the small pores increases proportionately to the vapour pressure difference; if the osmotic pressure exceeds the tensile strength of the pore walls, a crack develops. The cracks, in turn, become the new generation of small pores, advancing the deterioration process.
2.3 The effect of deicers
If the pores hold adsorbed deicer cations, the upper limit of the pore or capillary size that is filled with adsorbed water may increase several times. The amount of increase is a function of the concentration of the cations in the pore, the ionic size to charge ratio, and the clustering geometry of the water-ion system. Generally, the smaller the ionic radius and the ionic size to charge ratio, the greater is the water clustering ability of the cation. The net effect of deicer cations is to increase the number of pores and capillaries occupied by adsorbed water, and to increase the proportion of adsorbed water in the pore system. Thus a greater number of pores become osmotically active, and the deterioration is accelerated. The freezing and thawing of the pore system serves to concentrate the cations in the pores. The concentrated cations increase the osmotic pressure difference, and the severity of the deterioration.
|
Figure 1 Relationship of adsorbed water on the freeze-thaw loss of aggregates. |
The relationship between adsorbed water content and the degree of freeze-thaw deterioration is shown in Figure 1. The results shown are for a wide range of aggregates used in concrete in Canada. They include all the usual rock types: limestone, dolomite, granite, diorite gabbro, gneiss, volcanics and argillite, more or less in their order of frequency of occurrence. The freeze-thaw deterioration was determined by saturating the aggregates in a 3% NaCl solution, and freezing them in air in 100% humidity conditions for 5 cycles - 16h freezing at -16oC, 8h thawing at room temperature (22oC). Adsorption was determined by exposing the oven-dried aggregate to 98% relative humidity (RH) conditions at 22oC for 72h and determining the weight gain.
Figure 1 shows a good correlation between freeze-thaw damage and water adsorption (R=.90). The effect of adsorbed water on the freeze-thaw durability is clearly demonstrated.
3. Reducing the deicer salt damage - results of experiments
Assuming the above scenario of deicer salt damage is correct, then to reduce the physical deterioration due to the fine pore system requires that the osmotic differential among the pores be reduced. The osmotic difference will be reduced if the proportion of adsorbed, low vapour pressure water is reduced.
Two potential remedies are suggested: 1. Satisfy the cations of the current deicer salts (NaCl) by substituting a strong anion that will preferentially bind to the cation at the expense of the water molecule, thus reducing the amount of adsorbed water; or 2. Develop a deicer that does not cluster water molecules and does not increase the adsorbed water content. The first suggestion can be accomplished by admixing a large ionic size, strongly electronegative anion salt to NaCl or CaCl deicer. The second remedy requires testing a variety of salts for their effectiveness in melting ice without causing scaling damage to the porous materials.
3.1 Preventive treatments with existing deicer (NaCl)
Numerous chemical compounds were tested for their effectiveness as NaCl additives. The compounds were chosen mainly on the basis of their active anion, with the theory that the anion would bond with the adsorbed Na cation and interfere with water adsorption. Some of the major anion groups tested were nitrates, borates, other chlorides, and phosphates. The latter was found to be most effective, especially the mono cation phosphates: mono sodium, potassium, and calcium phosphates. Ammonium phosphates were also effective.
3.1.1 Salt Treatment
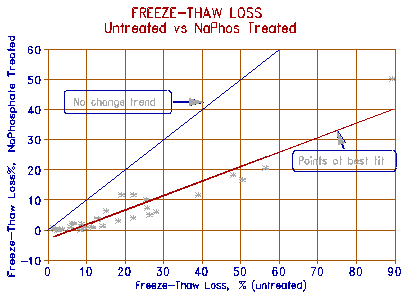
|
Figure 2 Reduction in freeze-thaw damage by 1:1 admixture of Na phosphate to NaCl. |
|
Figure 3 Reduction of freeze-thaw damage by 1:1 admixture of acidified mono calcium phosphate to 5% NaCl. |
Figures 2 and 3 show the effect of mixing 5% of NaCl with 5% of the mono sodium and mono calcium phosphates respectively. The salts were dissolved in water, aggregates saturated, and the freeze-thaw test was carried out as described above. The results are compared to the 'untreated' solution, i.e., one containing only 5% NaCl. The figures show that the Na phosphate - salt mixture significantly reduced the freeze-thaw breakdown; Ca phosphate was somewhat less effective. The difference in effectiveness may be due to the almost infinite solubility of Na phosphate, whereas Ca phosphate is insoluble under normal pH conditions, and can only be dissolved in an environment of pH of less than 3. Upon contact with reducing conditions (such as carbonate aggregate), the phosphate precipitates as a film in the pores. This may confer a longer-lasting protection, as will be seen later.
3.1.2 Treating Aggregates
|
Figure 4 Aggregate pre-treated in mono calcium phosphate prior to freeze-thaw test in 5% NaCl. |
|
Figure 5 Effect of length of treatment in mono calcium phosphate solution on freeze-thaw resistance. |
In some experiments, the aggregates were immersed in the Ca phosphate solution briefly, dried, and then saturated in a 5% NaCl test solution. The treatment was also effective in reducing the freeze-thaw damage, as shown in Fig. 4. Phosphate treatment is capable of 'beneficiating' or improving the performance of a marginal quality aggregate. The period of exposure to the phosphate solution has an effect on the results, as shown in Fig. 5. The results of ammonium phosphate are displayed here; similar results were obtained with calcium phosphate. The figure shows that, although more prolonged exposure to the 20% phosphate solution provides increasing protection, most of the protection is conferred within the first minute of immersion of the sample. The short exposure time would make the phosphate treatment fit easily into an aggregate plant process.
If treatment of aggregates to reduce the freeze-thaw damage in the presence of deicers is viable, the long-term effectiveness of the treatment needs to be determined. The main process to reduce effectiveness will be leaching of the treatment salt. A leaching experiment was set up during which treated aggregate, after drying, was washed in running water for specified time periods, dried, washed again, and after three cycles of washing, tested for freeze-thaw resistance. The experiment was designed to simulate three periods of rain with intervening drying periods.
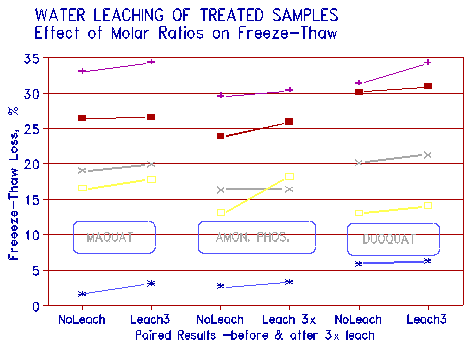
The results are shown in Figure 6. The figure shows that a nominal loss in the effectiveness of the treatment was detected. The figure also gives results for some of the other treatment chemicals tested in addition to phosphate.
All of the above treatments were applied on 'fresh' aggregate. Can a salt contaminated material be improved by treatment? Figure 7 shows the results of experiments designed to answer this question. Aggregate was first immersed in a 5% NaCl solution for 24h, dried, and immersed in a solution of ammonium phosphate of different concentrations for 5 minutes. After treatment, the aggregate was dried, and re-immersed in the 5% NaCl solution for 24h and subjected to freeze-thaw testing.
|
Figure 6 Leaching of aggregates after treatment - effect on freeze-thaw resistance. |
|
Figure 7 Treatment of salt-contaminated aggregate - effect on freeze-thaw resistance. |
The results show that phosphate is effective in reducing freeze-thaw damage of salt-contaminated aggregate. This finding has wider implications: i.e., salt-contaminated concretes could be 'beneficiated' by treatment with soluble phosphate solutions, and made more resistant to spalling and scaling due to freezing and thawing. Experiments along these lines are continuing.
3.1.3 Summary
The results presented above suggest that adding treatment chemicals to deicing salt, or treating the aggregate with the chemicals has a beneficial action in controlling the deterioration of the material due to freezing and thawing in a NaCl environment. Phosphates, either Na or Ca monophosphate, are the treatment chemicals shown to be the most effective.
Phosphates are well known as corrosion inhibitors. Rather simple experiments have shown that even a small amount of phosphate in a NaCl solution is effective in reducing rust formation. Calcium monophosphate is insoluble in water, but slightly soluble in NaCl solution. Sufficient phosphate anion may be dissolved in deicer water to reduce the rebar corrosion.
4. Alternate Deicers
Chloride-based deicers as a group are corrosive to concrete, embedded steel, and automobiles and are damaging to the environment. In the last several years, a search has been conducted for a non-corrosive deicer to replace salt; a leading contender has been calcium magnesium acetate (CMA)(1,2,3,4). During the winter of 1986-87, CMA was used as a deicer on selected roads in Ontario (Canada) and in three U.S.A. states (California, Massachusetts, and Wisconsin) (5). Although CMA was found to be an effective deicer, it is somewhat slower acting than NaCl, and at temperatures below -4oC it takes a long time to start noticeable melting; below -9oC no melting takes place without traffic. In addition to being less effective, CMA currently costs approximately 20 times more than NaCl.
Research in our laboratories has shown that a deicer of the same acetate family - a potassium acetate (KAC) - while possessing the same favourable properties of CMA is similar in its effectiveness to calcium chloride which is known as one of the most effective deicers at low temperatures.
4.1 Melting Rates of Potassium Acetate (KAC)
As part of a study to compare the various deicers, established and potential, a melting rate study was carried out. The study consisted of applying a measured quantity of the deicer, usually 20g, to a standard quantity of ice, usually 200g, at a given constant temperature of freezing. The temperatures chosen were -5, -10, -15, and -20 degrees centigrade. The amount of ice melted was measured at 15, 30, 45, and 60 minute intervals.
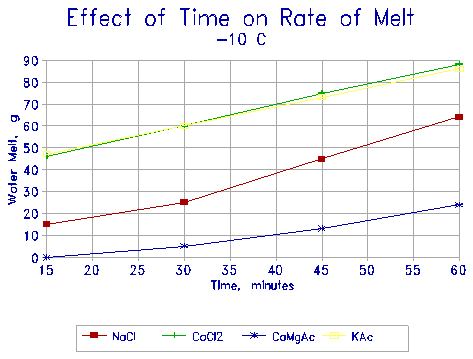
|
Figure 8 Melting rate of ice under various deicers at -10oC as function of time. |
|
Figure 9 Melting rate of ice under various deicers at -20oC as function of time. |
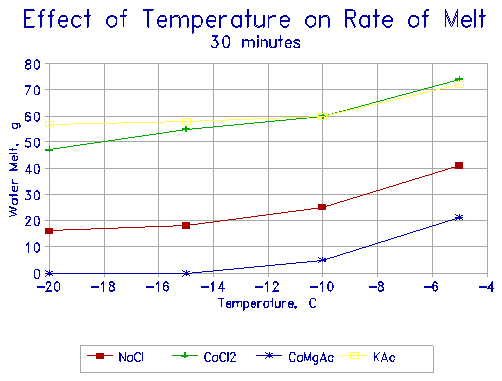
The results are presented in the next four figures (Figures 8 through 11). Four deicers are compared: NaCl, CaCl2, CMA, and KAC. Figures 8 and 9 show the melting rates of the four deicers at -10oC and at -20oC respectively as a function of time. It is seen that KAC is equivalent in its melting rate to CaCl2, and about twice as effective as NaCl. CMA, on the other hand, is very ineffective at -10oC - it takes almost 30 minutes to initiate any melting. It does not melt any ice at -20oC.
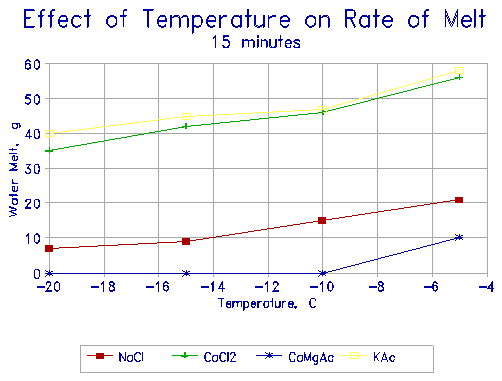
|
Figure 10 Effect of temperature on the melting of ice after 15 minutes exposure to deicers. |
|
Figure 11 Effect of temperature on the melting of ice after 30 minutes exposure to deicers. |
Figures 10 and 11 show the effect of temperature on the rate of melting. KAC and CaCl2 behave similarly, and melt almost 3 times as much ice as NaCl at any given temperature. CMA melts only small quantities of ice over the equivalent period, and virtually none below -10oC.
4.2 Scaling tests
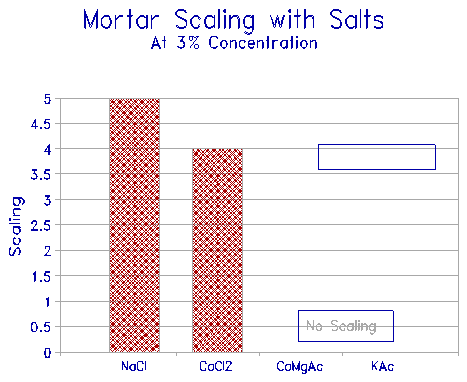
The scaling of mortars exposed to some of the above deicers were studied. The scaling was determined as follows: At age of 28 days, triplicate samples of two inch cube mortars were placed on a carpet saturated in the 3% test solutions in an air-tight container. The closed container was frozen at -16oC for 16h, and thawed for 8h at room temperature (22oC) for 5 cycles. The samples were then visually rated for deterioration of the face in contact with the saturated solution. A rating system of 0 to 5, where 5 is the full face deterioration, was used to rate the scaling potential of the solution. The results show (Fig. 12) that the acetates caused no scaling, while the chlorides, as expected, showed severe scaling. Thus, KAC, while exhibiting strong deicing capabilities, shows none of the deleterious effects associated with the chloride deicers. Although metal corrosion tests have not been carried out with KAC, it should not behave differently from CMA - i.e., it should be non-corrosive. The scaling test used was non-standard; however, the comparative results should be valid.
|
Figure 12 Scaling of mortar cubes exposed to various deicers. |
4.3 Relationship of scaling, de-icing effectiveness, and water adsorption
A deicer must be hygroscopic and hydrophillic at temperatures in the freezing range to be effective. Tests were carried out to determine the amount of water adsorbed at -3oC and at room temperature (22oC). Mortar cubes saturated for 24h in the various deicer solutions were dried, and exposed to 95% humidity at 22oC in a humidity chamber. After 72h, the gain in mass due to water sorption was measured.
|
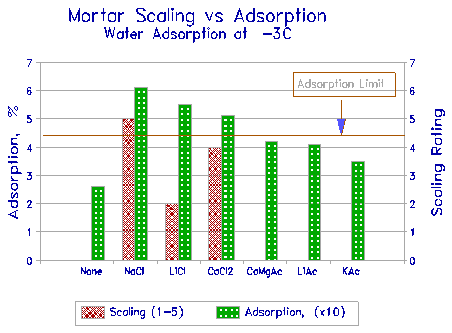
Figure 13 Water adsorption and scaling of mortar cubes exposed to various deicers. |
The results at -3oC, combined with scaling rating, are shown in Figure 13. The figure shows that the chloride-saturated mortars show the greatest water adsorption, and also the greatest scaling. None of the acetates show scaling, and they also show reduced water adsorption compared with the chlorides. As a suggestion, a limit is proposed to the maximum water adsorption above which scaling is likely to take place. There is no theoretical basis for this at present. When the deicing property of KAC is better understood, an explanation may be evident.
5. Summary and Conclusions
There are four ways of controlling the damage caused by deicers:
1. Do not use deicers. Control ice by sanding; however, sanding tends to plug drains.
2. Use additives to common chloride deicers to minimize their deleterious effects. Phosphates, especially Na and Ca monophosphates, have been shown to be effective in reducing scaling (and corrosion) due to use of NaCl as a deicer.
3. Use non-chloride deicers. Calcium magnesium acetate has been shown to be effective at near freezing temperatures, but ineffective at lower temperatures. The deicer proposed in this paper, potassium acetate, is as effective as the best chloride deicer, but in common with other acetates, is benign to concrete and the environment.
6. Acknowledgement
The work reported in the paper has been supported by an operating research grant to the two senior authors from the National Science and Engineering Research Council (NSERC) of Canada.
7. References
1. S.A. Dunn and R.U. Schenk, "Alernative Highway Deicing Chemicals", Transportation Research Board Spec. Report 185, TRB, NRC, Washington, D.C., pp. 261-269, 1979.
2. D.D. Ernst, G. Demish and T. Wieman, "Calcium Magnesium Acetate in Washington State", Transportation Research Record 1019, GTRB, NRC, Washington, D.C., pp. 8-12, 1985.
3. M.T. Hsu, "Production and Testing of Calcium Magnesium Acetate in Maine:, Transportation Research Board Record 962, TRB, NRC, Washington, D.C., pp. 77-82, 1984.
4. R.R. Horner, "Environmental Monitoring and Evaluation of Calcium Magnesium Acetate", NCHRP Report 305, TRB, NRC, Washington, D.C., April, 1988.
5. B.H. Chollar, "Field Evaluation of Calcium Magnesium Acetate During the Winter of 1986-87", Public Roads v52, n1, pp.13-18, June, 1988.