FREEZING OR OSMOSIS AS DETERIORATION MECHANISM OF CONCRETE AND AGGREGATE?
Peter P. Hudec
Geology Department, University of Windsor, Windsor, Ont. Canada
Abstract
Although concrete and aggregate deteriorate at an accelerated rate when exposed to freezing, and especially in the presence of de-icing salts, it is not all certain that the crystallization of ice is the principal destructive mechanism. Materials with majority of very fine pores are most susceptible to 'frost' deterioration, yet theoretically (and from experimental data), the water in these pores remains unfrozen in the normal range of freezing temperatures. De-icing salt decrease the freezing point and the amount of water freezing, yet acerbate the damage. Further, a 3 to 5 percent concentration by weight of de-icing salt is more damaging than lesser or greater concentrations.
An explanation may come from soil mechanics and clay-water system studies. A water film up to 0.3 m in thickness covers a clay, silica, or other mineral surface. In the presence of cations, the thickness increases as a function of the cation size/charge ratio (the Diffuse Double Layer theory). Thus, a pore of 0.3 m radius is completely filled with adsorbed water; adsorbed de-icing salt cations cause larger pores to fill, and create increased osmotic differential in the smaller pores. The pore tries to imbibe water from larger pores and capillaries, swells, and exerts pressure on water in the larger capillaries. If no relief to the hydraulic stress (in the form of empty larger pores or entrained air voids) is available, the material fails.
Freezing accelerates the process by increasing the osmotic difference. Assume a porous system saturated with 3% NaCl solution. During freezing, the solution in the larger pores freezes, and concentrates the NaCl in the small pores containing unfrozen solution. This causes greater adsorption of the Na+ ion on pore walls. On thawing, water in the larger pores, now depleted in the Na cation, is driven by osmosis into the smaller pores. Concentrations of NaCl greater than 5% decrease the osmotic potential between large and small pores; this explains why NaCl and other de-icers, including alcohol, are most destructive at low concentrations. It also explains why large ions such as phosphate and acetate (as in calcium magnesium acetate) reduce the freeze-thaw damage.
Thus, both freezing and osmosis play a part in the deterioration of porous materials saturated with de-icing salt solutions. The results of laboratory experiments supporting the above theory will be presented.
Hudec, Peter P., 1991, Freezing or osmosis as deterioration mechanism of concrete and aggregate? Proc. 2nd Workshop on Low Temperature Effects on Concrete, NRC, pp.1-8.
![]()
1. Introduction
Frost action damage clearly implies that freezing is the cause of physical breakdown of saturated or semi-saturated porous materials. The obvious cause of the breakdown during freezing is the 9% expansion of water phase to ice phase - the so-called milk-bottle effect. If the pore is critically saturated, i.e, water occupies more than 91% of its volume, the water, upon freezing, will exert pressure upon the pore walls, or on the surrounding unfrozen water in the finer pores. The latter is the basis of Power's theory (&&) of hydraulic pressure as major cause of deterioration during freezing and thawing. Litvan (&&) argued, and showed experimental evidence of water exuding the aggregate particle and freezing on the outside interface (on an unconfined, free-standing sample). No corroborative evidence of fracturing at the aggregate-paste interface in saturated frozen concrete has been observed. However, the experiments do indicate that the water does move under conditions of freezing - perhaps due to the hydraulic pressure, or due to other forces.
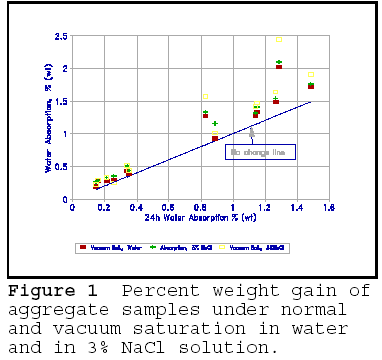
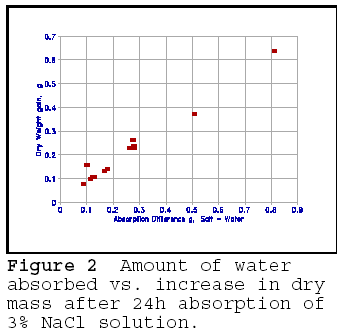
Deicing salts significantly accelerate the breakdown due to freezing and thawing. Salts of any kind dissolved in water reduce the freezing point, and the amount of water frozen. Deicing salts, most commonly NaCl, are no exception. Thus, an apparent contradiction exists: deicing salts reduce the amount of water freezing, yet cause more damage. It has been suggested that deicing salts increase the degree of saturation; this has been borne out by experiments. Figure 1 shows the 24h absorption of water of rock aggregate as compared to vacuum saturation, to 24h absorption in 3% NaCl solution, and vacuum saturation in the 3% NaCl solution. The figure shows a clear increase in the 24h absorption in NaCl solution, which is almost equivalent to vacuum saturation in plain water. However, before jumping to conclusions, note that the vacuum saturation in the 3% NaCl solution gives highest weight gain. Vacuum saturation in the salt solution could not have created more space; the increase in water absorbtion of porous material 'contaminated' with a solution of NaCl as calculated by percent increase in mass is largely due to the increase in the contents of solid salt present in the pores. Figure 2 illustrates the difference in absorption on the same rock specimen saturated for 24h in water and in 3% NaCl solution, and the difference in dry weight gain of specimen before and after saturation in 3% NaCl solution. As can be seen, the dry weight gain is almost equivalent to the increase in absorbtion in salt solution. The figure conclusively shows that salt in the rock pores does not cause significantly greater absorption, and thus saturation of the pores. There may be an increase in the number of pores that are 'critically saturated, but these are likely to be the very small pores in which water does not freeze.
 It is well known that the very fine grained sedimentary rocks such as
argillaceous (clay-rich) carbonates, shale, and siltstone are prone to rapid
deterioration. The deterioration of some these rocks can be effected by simply
wetting and drying. Freezing and thawing causes a very rapid breakdown,
especially in salt solutions. Fine size of mineral particles implies that the
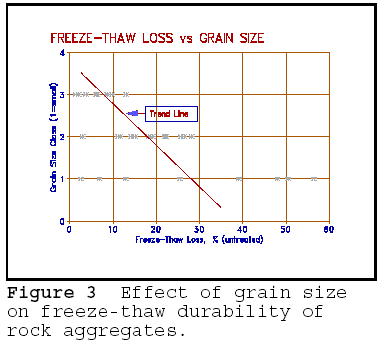
spaces or pores between the particles are also fine. Figure 3 shows a plot of
freeze-thaw resistance of rocks compared to visual estimate of grains size. The
grain size has been arbitrarily classified as small or fine (1), to large (3).
The classification is clearly crude, but serves to illustrate the point. All
large grained (large pored) rocks show minimal freeze-thaw damage, whereas the
fine grained rocks show both large and minimal damage. The wide range is
interpreted to be caused by few larger grains or pores existing in the rock. The
few large pores act as 'air entraining' in concrete.
It is well known that the very fine grained sedimentary rocks such as
argillaceous (clay-rich) carbonates, shale, and siltstone are prone to rapid
deterioration. The deterioration of some these rocks can be effected by simply
wetting and drying. Freezing and thawing causes a very rapid breakdown,
especially in salt solutions. Fine size of mineral particles implies that the
spaces or pores between the particles are also fine. Figure 3 shows a plot of
freeze-thaw resistance of rocks compared to visual estimate of grains size. The
grain size has been arbitrarily classified as small or fine (1), to large (3).
The classification is clearly crude, but serves to illustrate the point. All
large grained (large pored) rocks show minimal freeze-thaw damage, whereas the
fine grained rocks show both large and minimal damage. The wide range is
interpreted to be caused by few larger grains or pores existing in the rock. The
few large pores act as 'air entraining' in concrete.
It has been shown both experimentally and thermodynamically that the water in the small pores in these rocks does not freeze within the normal freezing range (to -20oC), and that the salt solutions decrease the amount of water freezing in all porous materials. If there is no freezing, or the amount of water freezing is reduced, then obviously there must be a decrease in the hydraulic stress caused by freezing. Yet salt solutions increase the freeze-thaw (and wetting-drying) breakdown. To account for this discrepancy, there must be other forces at work. The remainder of the paper will be devoted to proposing an alternate process of porous material breakdown which alone, or in combination with hydraulic freezing force process, contributes to porous material breakdown.
2. Osmotic Theory of Freeze-Thaw
Deterioration
2.1 Nature of pore surface:
All surfaces, including mineral surfaces, posses a surface charge; the amount of charge is a function of the nature of the surface. In minerals that make up the rock used as natural aggregate, the surfaces can be mineral faces (rarely), cleavage faces, and fracture faces. The latter are the most active, since fracture surface breaks randomly across chemical bonds of the mineral; cleavage face represents a direction of minimum bonding between elements of the crystal and are less active. Furthermore, if the crystal is 'imperfect', i.e., has an incomplete lattice, or a lattice in which under-or over-size elements substitute, or has a lattice that has been tectonically strained, the surfaces of that mineral will possess a greater charge. The charge on the surfaces is generally negative. The negative surfaces attract polar water molecules. The action is similar to that of sorption of cations onto clay particles - the "double layer" theory.
2.2 Pore Size
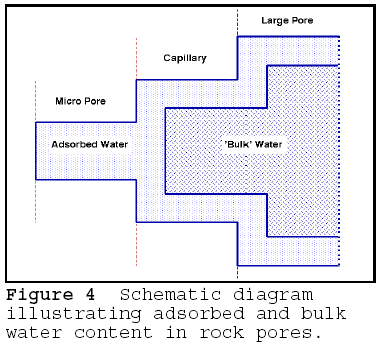 The
size of the pore determines the proportion of the adsorbed vs. 'normal' water
held in the pore. The water adsorbed at close to 100 percent relative humidity
at 20C completely fills capillaries of up to 5 um radius, as shown by the Kelvin
equation. The schematic illustration of the effect of pore size on the type of
water held is given in Figure 4. If the material contains greater proportion of
small size pores in the 'critical' range, more will be filled with adsorbed
water and the ratio of adsorbed to absorbed water in the porous material
increases. The adsorbed water in the pores has lower vapour pressure; the vapour
pressure of the water is proportional to the pore radius - thus, very small
pores have lower vapour pressure than do larger pores. If the porous material is
fully saturated by immersion, the larger pores contain mostly bulk, normal
vapour pressure water, and a vapour pressure potential exists between large and
small pores. Because of this difference, higher vapour pressure water is
compelled to move towards lower vapour pressure water by osmosis. The pressure
in the small pores increases proportionately to the vapour pressure difference;
if the osmotic pressure exceeds the tensile strength of the pore walls, a crack
develops. The cracks, in turn, become the new generation of small pores,
advancing the deterioration process. The expansive force created by the osmotic
flow can be directly measured: dry, fine grained rocks, when placed in water,
expand; when dried, they contract. Desiccation and re-wetting of clay is a very
similar process. This explains how very fine grained, fine pored rocks such as
shale deteriorate by wetting and drying alone.
The
size of the pore determines the proportion of the adsorbed vs. 'normal' water
held in the pore. The water adsorbed at close to 100 percent relative humidity
at 20C completely fills capillaries of up to 5 um radius, as shown by the Kelvin
equation. The schematic illustration of the effect of pore size on the type of
water held is given in Figure 4. If the material contains greater proportion of
small size pores in the 'critical' range, more will be filled with adsorbed
water and the ratio of adsorbed to absorbed water in the porous material
increases. The adsorbed water in the pores has lower vapour pressure; the vapour
pressure of the water is proportional to the pore radius - thus, very small
pores have lower vapour pressure than do larger pores. If the porous material is
fully saturated by immersion, the larger pores contain mostly bulk, normal
vapour pressure water, and a vapour pressure potential exists between large and
small pores. Because of this difference, higher vapour pressure water is
compelled to move towards lower vapour pressure water by osmosis. The pressure
in the small pores increases proportionately to the vapour pressure difference;
if the osmotic pressure exceeds the tensile strength of the pore walls, a crack
develops. The cracks, in turn, become the new generation of small pores,
advancing the deterioration process. The expansive force created by the osmotic
flow can be directly measured: dry, fine grained rocks, when placed in water,
expand; when dried, they contract. Desiccation and re-wetting of clay is a very
similar process. This explains how very fine grained, fine pored rocks such as
shale deteriorate by wetting and drying alone.
2.3 The effect of deicers
If the pores hold adsorbed deicer cations, the upper limit of the pore or capillary size that is filled with adsorbed water may increase several fold. This is illustrated in Figure 5. Compared to Figure 4, the deicer cations have caused both the micro pore and the small capillary to be occupied by lower vapour pressure water; the presence of cation in the micro pore probably lowers the vapour pressure of the contained adsorbed water, increasing the osmotic differential. The amount of vapour pressure increase, and the number of additional capillaries occupied by low vapour pressure water is a function of the concentration of the cations in the pore, the ionic size to charge ratio, and the clustering geometry of the water-ion system. Generally, the smaller the ionic radius and smaller the ionic size to charge ratio, the greater the water clustering ability of the cation. Thus, the net effect of deicer cations is to increase the number of pores and capillaries occupied by adsorbed water, and to increase the proportion of adsorbed water in the pore system. Thus a greater
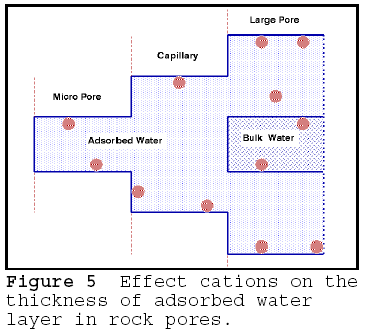
 number
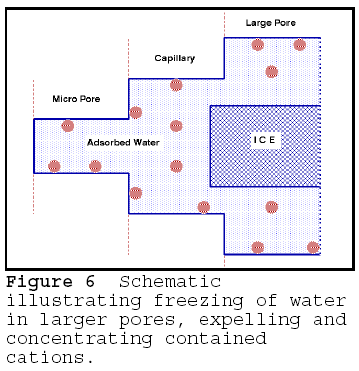
of pores become osmotically active, and the deterioration is accelerated. The of
freezing the pore water concentrates the cations in the pores, as shown in
Figure 6. The bulk water in the larger pores that can freeze expels the cations
from the ice crystal lattice into the unfrozen solution in the smaller pores.
The pore solution in the smaller, unfrozen pores becomes more concentrated.
Thawing of ice provides a higher vapour pressure water which resides in the
larger pores. The osmotic pressure difference between the concentrated solution
and the 'outside' world of larger voids and capillaries increases. This in turns
increases the severity of the deterioration.
number
of pores become osmotically active, and the deterioration is accelerated. The of
freezing the pore water concentrates the cations in the pores, as shown in
Figure 6. The bulk water in the larger pores that can freeze expels the cations
from the ice crystal lattice into the unfrozen solution in the smaller pores.
The pore solution in the smaller, unfrozen pores becomes more concentrated.
Thawing of ice provides a higher vapour pressure water which resides in the
larger pores. The osmotic pressure difference between the concentrated solution
and the 'outside' world of larger voids and capillaries increases. This in turns
increases the severity of the deterioration.
The relationship between adsorbed,
unfreezable water content and the degree of freeze-thaw deterioration is shown
in Figure 7. 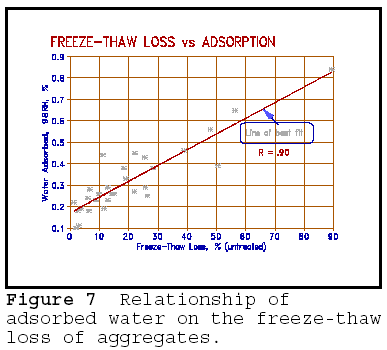 The results shown
are those for a wide range of aggregates used in concrete in Ontario. They
include all the usual rock types: limestone, dolomite, granite, diorite gabbro,
gneiss, volcanics and argillite, more or less in their order of frequency of
occurrence. The freeze-thaw deterioration was determined by saturating the
aggregates in a 3% NaCl solution, and freezing them in air in 100% humidity
conditions for 5 cycles - h freezing at -c, h thawing at room temperature (c).
Adsorption was determined by exposing the oven-dried aggregate to 98% relative
humidity (RH) conditions at c for h and determining the weight gain.
The results shown
are those for a wide range of aggregates used in concrete in Ontario. They
include all the usual rock types: limestone, dolomite, granite, diorite gabbro,
gneiss, volcanics and argillite, more or less in their order of frequency of
occurrence. The freeze-thaw deterioration was determined by saturating the
aggregates in a 3% NaCl solution, and freezing them in air in 100% humidity
conditions for 5 cycles - h freezing at -c, h thawing at room temperature (c).
Adsorption was determined by exposing the oven-dried aggregate to 98% relative
humidity (RH) conditions at c for h and determining the weight gain.
The figure shows a good correlation between freeze-thaw damage and water adsorption (R=.95). The effect of adsorbed, non-freezable water on the freeze-thaw durability is clearly demonstrated.
2.4 Most damaging deicer concentration
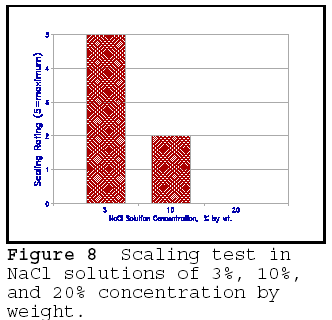 Tests have shown that the most
damaging NaCl deicer solution concentration is between 3 and 5 percent by
weight. This is shown in Figure 8, in which the results of the scaling tests on
mortar cubes are given in NaCl solution of 3%, 10%, and 20% concentration. It is
seen that the 20% concentration resulted in no scaling; freeze-thaw tests in 20%
solution give similar results to freeze-thaw tests in water. Similar
concentrations have been found for glycol, alcohol, and other chloride salts.
This 'pessiumum' concentration is difficult to explain by the hydraulic theory
of freeze-thaw failure. However, the osmotic theory gives an acceptable
explanation of the pessimum effect.
Tests have shown that the most
damaging NaCl deicer solution concentration is between 3 and 5 percent by
weight. This is shown in Figure 8, in which the results of the scaling tests on
mortar cubes are given in NaCl solution of 3%, 10%, and 20% concentration. It is
seen that the 20% concentration resulted in no scaling; freeze-thaw tests in 20%
solution give similar results to freeze-thaw tests in water. Similar
concentrations have been found for glycol, alcohol, and other chloride salts.
This 'pessiumum' concentration is difficult to explain by the hydraulic theory
of freeze-thaw failure. However, the osmotic theory gives an acceptable
explanation of the pessimum effect.
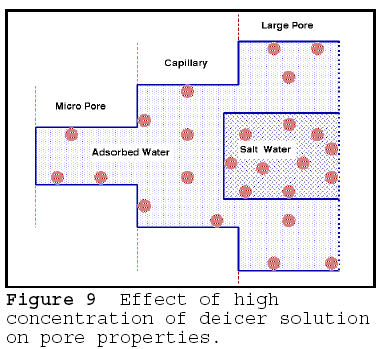 All surfaces have a potential
to adsorb ions. However, the sorption potential is finite. When the surface
reaches its saturation potential, it can no longer adsorb; it will in fact repel
additional ions of the same type. However, different, more aggressive ions or
different ions in greater concentrations will replace the first sorbed ions -
the well know concept of ionic exchange in soil science and clay mineralogy. The
concept can be applied to rock, mortar or concrete, as shown in Figure 9. The
finer pores and capillaries, because of surface effect, can contain only a
finite number of ions; however, the larger pores, away from the surface, can be
filled with highly concentrated solution. An osmotic flow from the finer to
coarser pores can result in shrinking or desiccation of the fine pores. The
effect can be readily seen in desiccation shrinkage of fresh water clays placed
in a brine solution. The shrinkage, however, is compressive, and acts against
the compressive strength of the rock; it is therefore well resisted, and does
not, in itself, cause problems. This explains why mortar shows almost no scaling
in 20% as shown in Fig. 8.
All surfaces have a potential
to adsorb ions. However, the sorption potential is finite. When the surface
reaches its saturation potential, it can no longer adsorb; it will in fact repel
additional ions of the same type. However, different, more aggressive ions or
different ions in greater concentrations will replace the first sorbed ions -
the well know concept of ionic exchange in soil science and clay mineralogy. The
concept can be applied to rock, mortar or concrete, as shown in Figure 9. The
finer pores and capillaries, because of surface effect, can contain only a
finite number of ions; however, the larger pores, away from the surface, can be
filled with highly concentrated solution. An osmotic flow from the finer to
coarser pores can result in shrinking or desiccation of the fine pores. The
effect can be readily seen in desiccation shrinkage of fresh water clays placed
in a brine solution. The shrinkage, however, is compressive, and acts against
the compressive strength of the rock; it is therefore well resisted, and does
not, in itself, cause problems. This explains why mortar shows almost no scaling
in 20% as shown in Fig. 8.
2.5 Osmotic vs Hydraulic Freezing Theory of Deterioration
The osmotic theory of failure suggests that the amount of adsorbed water in proportion to normal bulk water dictates whether the failure mechanism will be primarily due to osmotic forces, or due to freezing and hydraulic forces. If the pores are large enough, and contain mostly bulk water, water in them will freeze; if they are critically saturated, hydraulic stress will be set up.
 The proportion of adsorbed pure water in the rock relative to free void space
was found to be a good measure of the rock's freeze-thaw durability. Figure 10
is a triangular diagram in which total void space (as determined by vacuum
adsorption) is normalized to 1, and proportion of adsorbed to absorbed to
free void space is determined in terms of total void space. 'Critically'
saturated line is taken at 80% or 0.8. All samples falling below the line showed
high freeze-thaw losses. Within the critical zone, a vertical line separates the
samples; the samples to the right of the line contain most of their water in the
adsorbed state. No or little freezing of water was detected in this group; yet
this group of rock aggregates showed the greatest deterioration. The samples
falling in this corner are sorption or osmotic sensitive; the rocks falling in
the 'bulk' corner fail by freezing-generated hydraulic stresses.
The proportion of adsorbed pure water in the rock relative to free void space
was found to be a good measure of the rock's freeze-thaw durability. Figure 10
is a triangular diagram in which total void space (as determined by vacuum
adsorption) is normalized to 1, and proportion of adsorbed to absorbed to
free void space is determined in terms of total void space. 'Critically'
saturated line is taken at 80% or 0.8. All samples falling below the line showed
high freeze-thaw losses. Within the critical zone, a vertical line separates the
samples; the samples to the right of the line contain most of their water in the
adsorbed state. No or little freezing of water was detected in this group; yet
this group of rock aggregates showed the greatest deterioration. The samples
falling in this corner are sorption or osmotic sensitive; the rocks falling in
the 'bulk' corner fail by freezing-generated hydraulic stresses.
When exposed to deicing salts, the samples would migrate toward the adsorbed water corner - i.e., become more osmotically sensitive. Thus, deicing salts accelerate the failure due to osmosis at the expense of failure due to freezing-induced hydraulic stress.
3. Conclusions
Both osmotic and hydraulic processes of freeze-thaw deterioration are operating during freezing and thawing process. The relative dominance of each is mostly a function of pore size: if the material contains mostly small pores, the osmotic process dominates; if the dominant pore size is large, then the hydraulic forces dominate, assuming that the pores are critically saturated.
The deicing salts accentuate the osmotic process. Any deicing salts whose solution has low osmotic potential will cause less damage. Equivalent concentration of calcium magnesium acetate (CMA) in solution has significantly lower vapour pressure than NaCl, and CMA is a non-damaging deicer. Also, any additive to NaCl salt that lowers its osmotic potential causes the mixture to be less damaging, as shown in the companion paper.