COMMON FACTORS AFFECTING ALKALI REACTIVITY AND FROST DURABILITY OF AGGREGATES.
PETER P. HUDEC Geology Department, University of Windsor, Windsor, Ont., CANADA
Abstract
Petrographically similar rock aggregates are involved both in alkali reactivity and frost durability of aggregates. Examples are some cherts, argillaceous dolomites, and phyllites. Although alkali reactivity is essentially a chemical, and freeze‑thaw durability a physical process, it appears that some common factors affect both.
The two common factors that have been identified are: a. the very fine grain size and pore size, and the attendant high surface area, and b. highly active mineral surfaces. The active, commonly negatively charged surfaces attract alkali cations and polar water (and other) molecules. The concentration of alkalies in the small pores results in osmotic differential and pressure ‑ the cause of physical deterioration. The same concentration also initiates a chemical reaction if suitable chemistry exists (i.e., alkali ‑ silica or alkali ‑ carbonate reaction).
Results of alkali reactivity and freeze‑thaw durability experiments confirm the above relationship. Some aggregates degrade during unconfined freezing and thawing in the presence of de‑icing salts, and also expand as a result of accelerated alkali reactivity tests. Both expand isothermally on saturation. High internal surface area, and sorption of both water and ions are the common properties to non-durable and alkali reactive rocks. It should, however, be stressed that not all frost sensitive aggregates are alkali reactive, and not all alkali reactivity aggregates have frost durability problems.
Keywords: Aggregates, Frost durability, Alkali reactivity, Grain size, Pore size, Surface area, De-icing salts, Expansion, Adsorption, Internal surface area.
Hudec, Peter P., 1990, Common factors affecting alkali reactivity and frost durability of aggregates, Fifth Intern. Conf. on Durability of Building Materials and Components, Baker, J.M et al, eds., Chapman & Hall, New York, pp.77-86.
![]()
1. Introduction
Alkali reactivity of aggregates in concrete is basically a chemical reaction. In alkali silica reaction (ASR), the alkalies of primarily Na and K react with the siliceous aggregate to produce a silica gel (Hobbs, 1988). The gel fills available pores. Two main theories have been proposed to explain the expansion of the concrete: the pressure of the gel as it fills the pores (Vivian, 1950), and pressure due to osmotic difference between the water in the gel and outside of the gel (Hobbs, 1979). In the latter, the gel imbibes water, swells, and creates expansive stress on the pore walls. The concrete expands, and eventually fails in tension by microcracking. The larger the surface area of the reactive silica or silicate and greater its surface activity, the greater the production of the gel and expansion (assuming sufficient alkalies).
In the case of alkali-carbonate reaction (ACR), the reaction process is quite different. The most reactive carbonate rocks are partially dolomitized argillaceous limestones. In these rocks, the clay particles tend to be concentrated in the vicinity of the small dolomite rhombs. Process of de-dolomitization in the presence of strong alkaline solution is thought to be the cause, but not the reason for expansion (Swenson and Gillot, 1960). The expansion in argillaceous dolomitic limestone is attributed to water uptake by dry clay minerals enclosed originally in dolomite, but now exposed by the de-dolomitization reaction (Gillot and Swenson, 1969). The reactive carbonate rocks are characterized by very fine grain size, low absorption, and low porosity (Dolar-Mantuani, 1983). The adsorption of water into the very small pores causes expansive stresses within the rock, and eventual failure of concrete in tension.
In both ASR and ACR reactive rocks, the high internal surface area of the reactive minerals is primarily responsible for the initiation of the expansion. The alkali reactivity can also be initiated in presence of any alkali salts, not just NaOH. Road salt (NaCl) has been shown to initiate both types of reaction (Larbi and Hudec, 1990). NaCl is also well known as an accelerator of physical damage during wetting, freezing, thawing, and drying cycles. It can also be shown that the rocks that tend to deteriorate rapidly in the presence of salt under the above climatic cycles have large internal surface area and small pore and grain size (Hudec, 1989, 1987). This paper will show that indeed a relationship exists between freeze-thaw durability and alkali reactivity for some, but not for all rock types.
2. Common Sorption Properties of AR and Freeze-Thaw Sensitive Rocks:
|
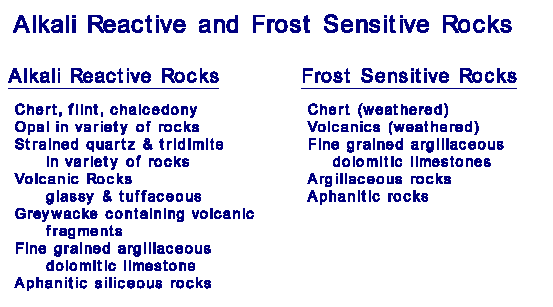
Table 1
gives the most common rock (and mineral) types that are alkali reactive,
and those that are frost and weathering sensitive. |
 |
To be alkali-silica reactive, the rock must, of course, contain silica or silicate. That is not a requirement for frost sensitive rocks. For ACR rock, the requirement is that it be fine grained, argillaceous, and dolomitic. All but the last appear to be the requirements for frost sensitivity among carbonates.
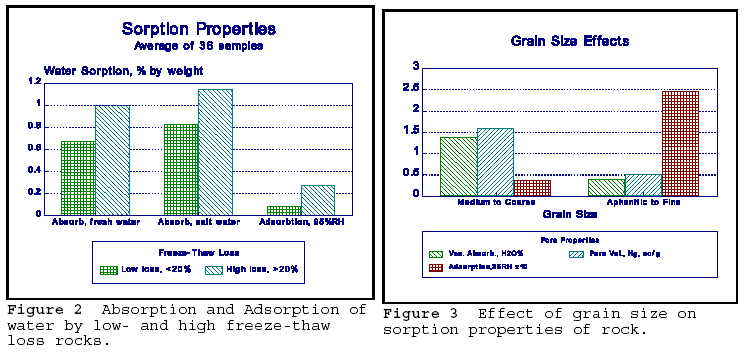
Fine grained to aphanitic texture is a common property for both AR and frost sensitive (FS) rocks. The fine grain of the minerals in the rock provides both larger surface internal area and small pore diameters. Figure 2 shows the effect of grain size, on vacuum absorption of water, injection volume of mercury, and the adsorption of water at 35% relative humidity (RH) for medium to coarse grained and aphanitic to fine grained group of rocks respectivelly. The rocks are mostly carbonates. Vacuum absorption and mercury intrusion give approximately the same results, and represent the total pore volume of the rock. Adsorption of water at 35% RH results in one to two layer coverage of the internal surface by the water molecule, and therefore gives a measure of the internal surface area. At 35%RH, water meniscus cannot exist, and here is no capillary filling; the water is adsorbed directly to the mineral surface. The
results clearly show that the fine grained rocks have over five times the internal
surface area of the coarser grained rocks. In AR, the increased surface area promotes reaction. In FS, the adsorbed water has lower vapour pressure, and acts as an osmotic pump for ingress of higher vapour pressure of bulk water. Expansion occurs under both conditions of AR and FS sensitivity.
The relationship between sorption properties and FS is further explained in Figure 3. The amount of water absorbed depends on the salinity of the water. In this case, more of the 3% NaCl solution was absorbed compared to fresh water in the same high and low FS rocks. NaCl is thought to concentrate by ionic sorption on the mineral surface, and attracts more water into the pore by osmosis. The concentration of alkalis at the mineral surface by adsorption will initiate the AR process under proper circumstances.
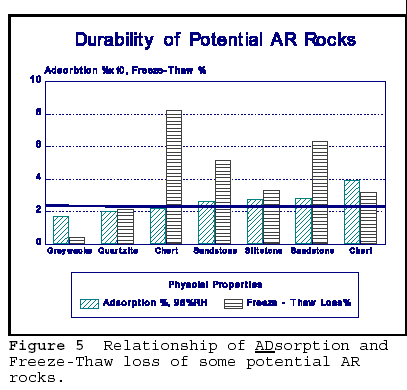
In Figure 4, some potentially AR reactive rocks have been selected for comparison of the physical properties that influence durability. The line drawn through the figure gives approximate limit above which the rock may be potentially alkali silica reactive, mainly because of its higher internal surface area as measured by adsorption. It is seen that the rocks above this limit also show significantly higher freeze-thaw losses. The above rocks have not been specifically tested for AR, but belong to the rock types known for their AR potential.
3. Expansive Properties of AR and FS Rocks:
The failure of concrete and rock by either AR or FS ultimately involves internal pressures and increase of volume. The AR is measured by monitoring the length change of the specimen as a function of time. Expansion also takes place during freezing-thawing cycles. The type of cracking observed in mass concrete
is virtually indistinguishable whether AR or FS is involved. Both produce 'map' cracking pattern. It is therefore instructive to consider the expansive potential of rocks suspected of AR and FS.
|
|
Water sorption properties were shown to influence FS and possibly AR in the previous section. NaCl, which is both an FS and an AR agent, is shown to
influence the sorption properties. Figures 5 and 6 illustrate the length change observed upon immersing the dry cores of rocks in water as compared to immersion in 3% NaCl solution. Some rocks contract upon wetting, others expand. Generally, those rocks that expand tend to be the frost sensitive ones, and some also AR sensitive. Both isothermal contractions and expansions upon wetting are equivalent to thermal expansions of several tens of degrees Centigrade - i.e., equivalent of rapidly heating or cooling the rock over this temperature range. Wetting and drying can significantly stress the rock and the surrounding concrete paste. The stress can results in the microcracking of both aggregate and paste, which allows greater access of solutions, and accelerates the breakdown process.
The difference in isothermal expansion on immersion in salt solution is also illustrated in Figures 5 and 6. The same rocks, after immersion in water were immersed (after bench drying) in a 3% NaCl solution, bench dried, then immersed in water, and finally, without drying, placed back in the salt solution. latter step resulted in net shrinking of the specimen. The results illustrate that the nature of the ionic solution in the pores affects the isothermal expansion or contraction of the rock. This is to be expected, since it is basically the osmotic difference between the pore water and the surrounding environment that drives the expansion or contraction of the system upon wetting. The presece of the de-icing salt solution changes not only the pore water chemistry, but the physical behaviour of the pore-water-rock-paste system. Both the physical changes (expansion and microcracking), and chemical changes (concentration of alkalies at the pore and microcrak walls) combine to produce the AR reaction.
|
|
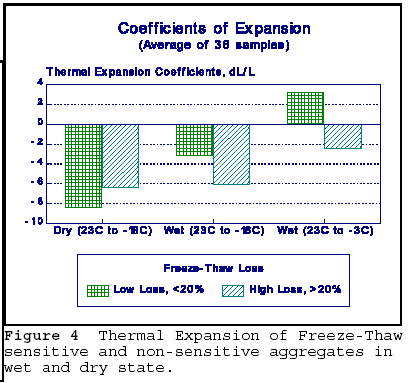
Figure 7 gives the average thermal coefficients of expansion for frost sensitive and non-sensitive rocks. It is seen that the thermal coefficients differ, depending on the saturation state of the rock, and whether the rock is allowed to freeze. The saturated coefficients are lower, perhaps because the rock has already undergone volume change on immersion. Lowest coefficients are observed when saturated rock is cooled to -3oC. This is likely due to the volume change experienced on wetting. The bars in the diagram represent the average length changes. Thus, in any one group there are both contractions and expansions experienced, and the value of the bars in the figure is the numerical average. As a result, the diagram has to be interpreted with some caution. However, the trends observed are valid.
|
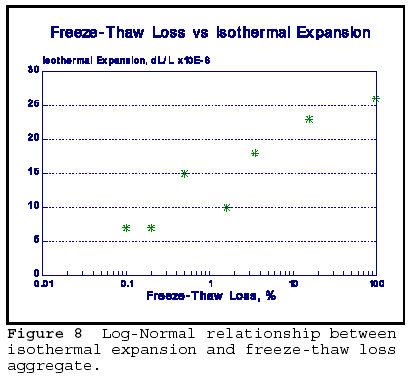
Figure Log-Normal relationship between isothermal expansion and freeze-thaw loss aggregate. |
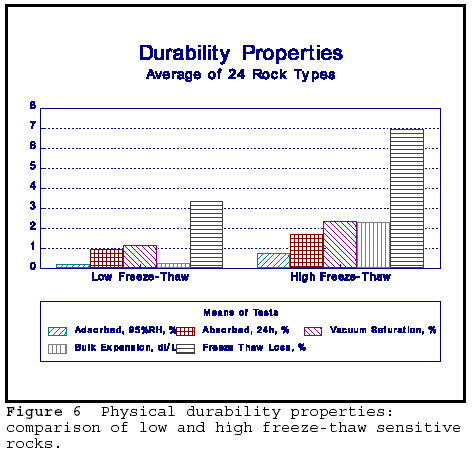
The summary of the physical durability properties are shown in Figure 8. Adsorption, absorption, vacuum saturation, bulk expansion and freeze thaw loss values are given for frost sensitive and non-frost sensitive rocks. The bulk expansion is isothermal expansion determined on a crushed, bulk materials by immersion in water. It can be seen that all values are higher for the FS rocks compared to non-frost sensitive rock. The rocks in this group represent a variety of rock types used as aggregates in SW Ontario. Sorption and isothermal expansion correlate well with freeze-thaw loss as measured by direct freezing and thawing test on unconfined aggregate. The test uses 3% NaCl solution as an accelerator for deterioration. That isothermal expansion is directly
|
|
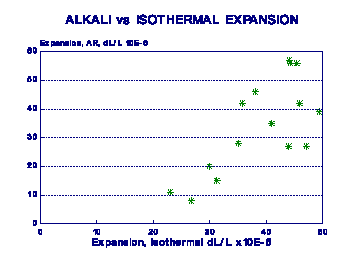
related to freeze-thaw resistance is shown in Figure 9. A strong log -normal relationship exists between the two parameters. A similar relationship can be expected between isothermal expansion and wetting-drying deterioration. The relationship suggests that even small isothermal expansions can result in significant physical deterioration, if sufficient number of either freezing and thawing or wetting and drying cycles are involved. Freezing can be considered as a drying cycle, since water is effectively removed from the system to form ice. The expansion due to ice formation may accelerate the process, but does not seem to be the dominant process.
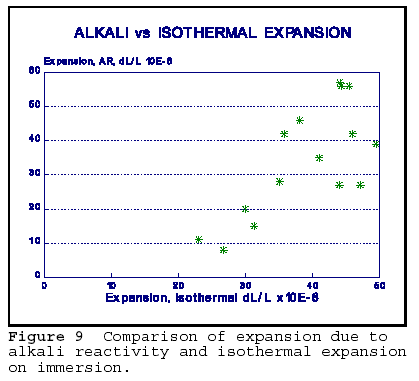
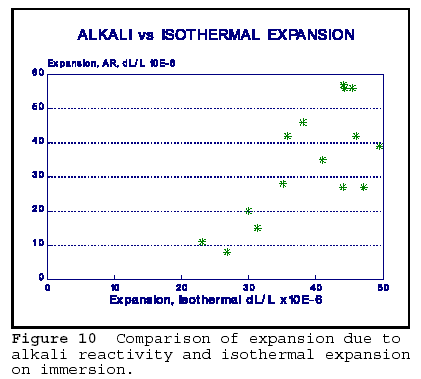
The last figure in this paper, Figure 10, strongly supports the connection between the expansion due to alkali reactivity and physical expansion due to
|
|
wetting. The data was obtained on cores of concrete containing various proportions and sizes of reactive chert aggregate. Before exposing the cores to the rapid alkali reactivity test, the isothermal expansion upon wetting was determined. Then, the concrete was subjected to 21 days of exposure to hot (80oC) 1N NaOH solution. It is interesting to note that the overall magnitude of expansions noted were similar for both expansive processes. It may be possible that isothermal expansion provides the space into which silica gel can migrate and continue the expansion as alkali reactivity proceeds. Isothermal expansion is reversible (with some hysteresis) upon drying; however, AR products may prevent the contraction on drying, and the concrete stays expanded.
4. Discussion and Conclusions.
The aggregates involved in alkali-silica and alkali-carbonate reaction are usually very fine grained, and therefore have very small pores. The fine grain results in high internal surface area. The high internal surface area is required for the AR reaction to proceed. High internal surface area was also found in aggregates that fail during freezing and thawing cycles, especially in presence of de-icing salts.
Expansion of the aggregates and concrete during AR and freezing and thawing process results in microcracking and eventual deterioration of the concrete. Adsorption of water and alkali ions on the internal surfaces of pores promote osmotic pressures that result in expansion and microcracking. Evidence was presented that aggregates that are frost sensitive have higher internal surface area, higher water adsorption and absorption, and expand more on wetting than do the more durable aggregates. Finally, it was shown that expansion of concrete containing alkali reactive aggregate when immersed in water or NaCl solution is similar in magnitude to expansion of the same concrete when subjected to accelerated alkaline reactive environment.
The results of this work indicate that isothermal expansion of aggregate, or concrete or mortar can be used as a quick screening method for detecting potential alkali reactivity.
The study was done on a limited number of alkali reactive aggregates, and must be expanded before the above observations can be universally applied to all AR materials.
6. Acknowledgement
The work described in this paper has been supported by grants from the National Research and Engineering Council of Canada (NSERC). Some of the experimental data has been gathered over the last several years by both undergraduate and graduate students working under author's direction. The financial support by NSERC and the diligent work by the students is gratefully acknowledged.
7. References
Dolar-Mantuani, L. (1983) Handbook of Concrete Aggregates. Noyes Publications, Park Ridge, N.J. USA.
. Gillot, J.E. and Swenson, E.G. (1969) Mechanism of Alkali-Carbonate Rock Reaction. Qart. J. Eng. Geology, 2, pp.7-23.
Hobbs., D.W. (1978) Expansion of concrete due to the alkali-silica reaction: an explanation. Mag. Concr. Res., 30, pp. 215-220.
Hobbs, D.W. (1988) Alkali-silica reaction in concrete. Thomas Telford Ltd., London, UK.
Hudec, P.P. (1987) Deterioration of aggregates - the underlying causes. Concrete durability. Katharine and Bryant Mather Int. Conference, Amer. Conc. Inst. SP-100, 2, pp. 1325-1342.
Hudec, P.P. (1989) Durability of rock as function of grains size, pore size, and rate of capillary absorption of water. Jour. Materials in Civil Engg. 1/1, pp. 3-9.
Larbi, J.A. and Hudec, P.P. (1990) A study of alkali-aggregate reaction in concrete: Measurement and Prevention. Cement and Concrete Research, vol. 20 (in press).
Swenson, E.G., and Gillot, J.E. (1960) Characteristics of Kingston Carbonate Rock Reaction. Highway Res. Board, Bull. pp. 275, 18-31.
Vivian, H.E. (1950) Studies in cement-aggregate reaction. XV: The reaction product of alkalis and opal. CSIRO, Melbourne, Australia, Bull. 259, pp.60-82.