IMPROVING AGGREGATE QUALITY BY CHEMICAL TREATMENT
Peter P. Hudec and Francis Achampong
Geology Department
University of Windsor
Windsor, Ont. Canada
N9B 3P4
Hudec, P.P., Achampong, F., 1994, Improving aggregate quality by chemical treatment; in Gerd W. Luttig, ed: Aggregates-- Raw Materials' Giant, 2nd Int. Aggregates Symp., Erlangen, Oct. 22-27, 1994., pp. 135-146.
Many fine grained aggregate types such as argillaceous carbonates, volcanics, sandstones, and chert and shale impurities degrade with repeated wetting and drying and with freezing and thawing, especially under the influence of de-icing salts. Surface coatings to prevent water and/or salts from entering the aggregate pores have been found successful, but expensive.
Chemical treatment of the internal pore surfaces by surface-active chemicals has proven effective in reducing aggregate (and concrete) deterioration. Large, electronegative anions such as phosphates and nitrates interfere with the adsorbed water layer and adsorbed de-icing salt (Na) ions, reducing osmotic pressure differential between the micro pores and larger pores. Large cations, such as those found in ammonium salts compete with Na ions for internal surface sites, and effectively disrupt the adsorbed water layer. Lowering the osmotic pressure reduces the cracking of the aggregate and concrete. Several chemicals were found to be effective in pre-treatment of aggregates, and as admixtures to de-icing salts, significantly reducing the aggregate and concrete deterioration.
It is possible to pre-treat the aggregate prior to use in concrete, treat the concrete, or mix the chemicals with de-icing salts and thus significantly reduce the deterioration of concrete surface. Laboratory test results showing the effectiveness of the various treatments are presented in the paper. The poorer the aggregate, the greater is the beneficiating effect of chemical treatment.
INTRODUCTION
The use of de-icing salts (largely NaCl) in the northern latitudes of North America has resulted in progressive deterioration of the concrete road infrastructure, especially the bridges. To minimize the damage, steps have been taken and specifications issued designed to make either the cement paste, the mortar, or the aggregate (or concrete as a whole) less prone to deterioration. Concrete paste can be made resistant to de-icing salt by air entrainment. However, the aggregate that makes up roughly 70% of the concrete must be generally accepted as is - after careful testing, and possibly physical beneficiation to remove the more harmful particles. The testing is not always sufficient to recognize the deleterious aggregate, and the conditions of testing are not specifically designed to simulate long-term exposure to and progressive saturation with de-icing salts.
The sources of aggregate with good service record are getting depleted, and new, acceptable aggregate sources are difficult to find and to develop, especially in the more urbanized regions that are the largest users of the material. There are two alternatives available to make better use of scarce resources and funds: make the concrete last longer, and use the material available, even if it is of lower quality. The two alternatives seem diametrically opposed. New technology is needed to bring them closer together.
Physical as means of improving the quality of the aggregate has been used successfully for years. It is based on the observation that most deleterious aggregate fragments are generally of lower specific gravity than the bulk of the 'good' aggregate. Heavy liquid suspension is the most common method of `floating' and separating the deleterious fraction. The method works on such materials as chert, shale, and weathered rock fragments. It is ineffective against the more subtly deleterious particles, such as very fine grained and argillaceous fragments of many rock types, but especially carbonates.
Surface coating of aggregate by polymers and oils (Cady et al, 1978, Ohama et al, 1987) has been suggested means of . The surface coating, to be effective, should not affect the bonding properties of aggregate, and should, preferably, be permeable to water but not to salt - a molecular sieve.
The suggested in this paper is a chemical treatment of internal surfaces of rock aggregates to make them less attractive to Na+ and other cations thought responsible for much of the deterioration. The freeze-thaw deterioration is accelerated by the de-icing salts - a seeming contradiction. The explanation of the deterioration process under de-icing salts, and means of preventing it is discussed in the next section.
THEORY OF DE-ICER DAMAGE
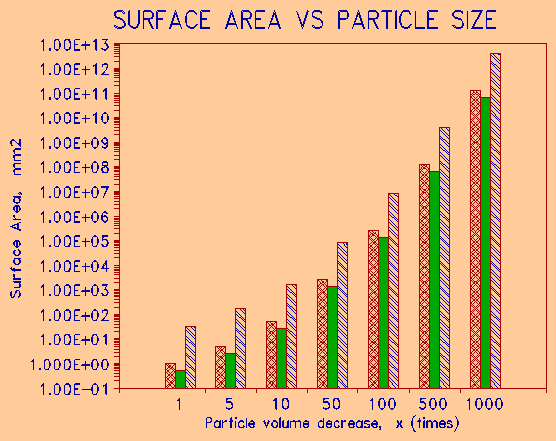
A classical paper on the relationship of the physical properties on the frost resistance of aggregates was written by Verbeck and Landgren in 1960. Since then it was found that the grain size, pore size, and total internal surface area of rock aggregates has a major influence not only on freeze-thaw durability, but also on wetting-drying resistance and overall durability of the aggregate. As the grain size decreases, the total surface area of the same mass of solid increases. No exact mathematical model can be used, since grains are irregular in shape. For illustration purposes, three shape end members could be considered: a cube, a square, and a platelet (flat rectangle). Table 1 shows how a unit mass of solid increases in surface area as the particle size decreases. The table is graphically represented in a log-normal diagram of figure 1. Note that the particle shape becomes less important as the particle size decreases - i.e., the total surface area is governed more by grain size than by grain shape. Internal surface area of a rock composed of fine particles plays a major role in aggregate and concrete deterioration (Hudec, 1989).
|
Table 1. SURFACE AREA AS FUNCTION OF GRAIN SIZE Grain Size (axes, mm) Size SURFACE AREA INCREASE (x) x y z Factor COMPARED TO 1 CC SOLID VOLUME Decrease Cube(y) Sphere(y) Plate(xyz) 10 10 0.1 1 1E+00 5E‑01 3E+01 2 2 0.02 5 5E+00 3E+00 2E+02 0.2 0.2 0.002 10 5E+01 3E+01 2E+03 0.004 0.004 4E‑05 50 3E+03 1E+03 8E+04 4E‑05 4E‑05 4E‑07 100 2E+05 1E+05 8E+06 8E‑08 8E‑08 8E‑10 500 1E+08 7E+07 4E+09 8E‑11 8E‑11 8E‑13 1000 1E+11 7E+10 4E+12 |
|
Figure 1: Graphical representation of Table 1. |
The physical breakdown observed in diverse materials is strikingly similar, starting with the development of microfractures and cracks. These may lead to complete disintegration of the material. Deterioration occurs only in the presence of water. Pure water was shown to be innocuous; it is the nature and the concentration of dissolved ions in the water that dictate the severity of deterioration. The general scenario of deterioration involving dissolved ions was summarized by Hudec (1989) and can be outlined as follows:
The pore surfaces possess residual charges, usually negative, the strength of which is the function of the degree of disorder in the crystal lattice of the minerals making up the surface. The surfaces attract the ions dissolved in pore water, concentrating them in layers along the surface. The small pores thus contain a higher concentration of ions relative to a larger pore; an osmotic differential may develop with attendant expansive and contractive forces. The expansive forces, acting against the tensile strength of the solid, may microfracture the solid, creating more fine pores. Changes in the conditions of exposure such as wetting, drying, freezing, and thawing affect the pore water ion concentration, and thus the magnitude of the osmotic force. These changes are reflected in the volume change of the solid. The solid particle itself may be disrupted, or, by virtue of expansion, exert disruptive forces on the enclosing matrix, fracturing it (as in the case of an aggregate particle in concrete).
Not all ions are equally disruptive. The cation with high charge/ionic size ratio, such as Na, behave more aggressively than a larger K ion. The aggressive behaviour of the ions is not restricted to creating destructive osmotic forces. Na and K cations are involved in an alkali‑silica and alkali‑carbonate reaction that has proven destructive to concrete.
The cation in the de-icing salt is thought to be preferentially attracted to the pore surface, and in turn attract the polar water molecules to itself. One way to decrease or eliminate the deleterious effects of salt cations is to either not allow them access to the pores (semi-permeable or impermeable membrane), displace them by other ions, or satisfy their charge, and thus minimize their attraction for water molecule. It is this latter approach that was chosen for the research described in the following sections.
If some ions are more disruptive than others, then replacing, complexing, or otherwise distracting these ions from the surfaces of pores may ameliorate their behaviour. One approach is to seal the surface so that no ion‑laden waters may reach the pores. Application of seals (mostly organic) has met with limited success. Another method may be to introduce other ion(s) that will interfere or compete with the aggressive ones. This was the approach taken in current research. Some phosphate compounds have been found effective in this function. The research is continuing to isolate other compounds that may also work.
EXPERIMENTAL APPROACH
General durability and response of aggregates to freezing and thawing can be determined by a variety of direct and indirect tests. Freezing and thawing of aggregates is perhaps the most direct way of determining their resistance to this climatic factor. Several tests exist for this purpose. The freeze-thaw test that gives the most consistent results, is relatively fast, and best approximates the conditions of freezing and thawing in service has been developed by the senior author, and is now used as a specifying test by the Ontario Ministry of Transportation (MTO, 1989, No. LS-614). The test is performed by first saturating the aggregate in 3% by weight of NaCl solution for 24h. The aggregate is then placed in a mason jar of sufficient size with 2 to 3 ml of solution remaining in the jar. The jar is sealed, placed on a side, and frozen for 16h at -18oC, and thawed at room temperature for 8h. The freeze and thaw cycle is repeated five times, the jar rotated 1/4 turn with each cycle. Loss is determined by back-sieving on the same size sieve. A more severe variation of the test is to place the aggregate on a saturated mat or sponge in a flat container rather than the jar.
Other tests employed in this study were water absorption, adsorption, specific gravity determination, grain size estimation, magnesium sulphate loss, and petrographic number determination (MTO, 1989, No. LS-609).
Effect of Grain Size:
|
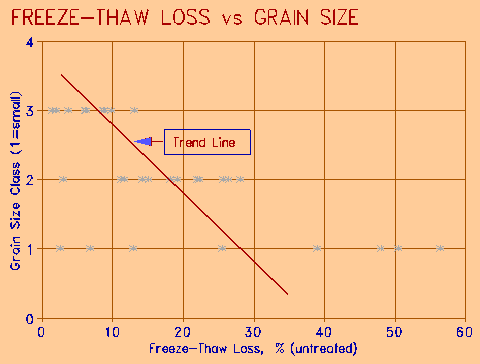
Figure 2 Relationship of grain size on Freeze-Thaw Loss. |
The grain size was visually categorized as 1 = small (fine), 2 = medium, and 3 = coarse. The results of the freeze-thaw test were plotted against these categories, and are shown in Figure 2. The coarse grain sized aggregate showed a uniform low loss; as the grain size decreased, the loss increased. The fine grained aggregate showed a wide range of losses, indicating that the grain size alone is not a good indicator of freeze-thaw resistance. It does, however, suggest that the fine-grained rocks are more prone to freeze-thaw damage.
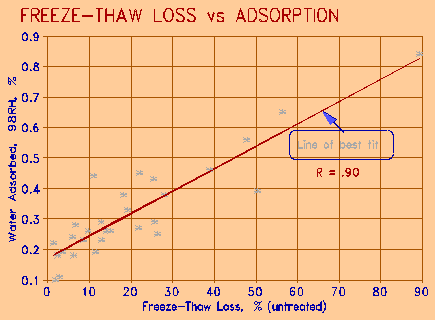
The grain size by itself has no effect on durability. It is the pore size related to grain size that does. Fine grain size produces fine pore size, which in turn produces large internal surface area for water and ion sorption. The relationship between water adsorbed at 98% relative humidity and at 23oC, and the freeze thaw loss is illustrated in Figure 3. A good correlation is seen, as expected from previous work by the senior author and others (Hudec, 1987). Water is adsorbed on the surfaces of the pore wall; if the pore is small enough, the adsorbed water can completely fill the pore. The pore thus contains water of lower vapour pressure than the water in the surrounding, larger pores, and becomes a centre of osmotic in-flow. Water adsorbed at the humidity of the experiment fills pores less than 1 Pm in diameter. The correlation suggest that more small pores the rock contains, the more prone it is to freeze-thaw damage. These pores can be termed "force pores", in that they are probably responsible for the expansive forces that expand and deteriorate the rock.
|
Figure 3 Freeze-thaw loss as function of water adsorption at 98% Relative Humidity, 22oC. |
|
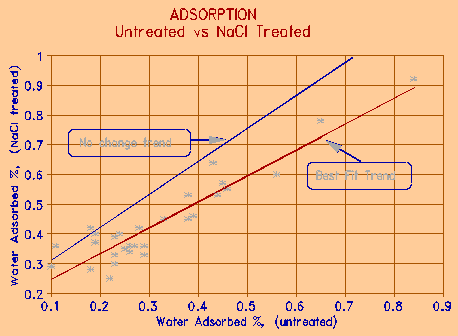
Figure 4 Relationship between water adsorbed before and after treatment with 3% NaCl salt solution. |
If the rock is saturated in the de-icing solution, dried, and then exposed to the same humidity conditions, the amount of water adsorbed increases. Figure 4 shows the relationship between water adsorbed on the same sample before and after treatment with 3% NaCl salt solution. It is seen that the salted specimens adsorb approximately 10% more water than the unsalted ones under same conditions of exposure. This implies that either 10% more pores are filled, or pores that are 10% larger in diameter are filled. The effect is to increase the number of "force pores", and therefore the amount of expansion the rock particle experiences. This, naturally, leads to greater deterioration. This is one explanation why the de-icing salts tend to be harmful even though they decrease the amount of water freezing in the rock.
|
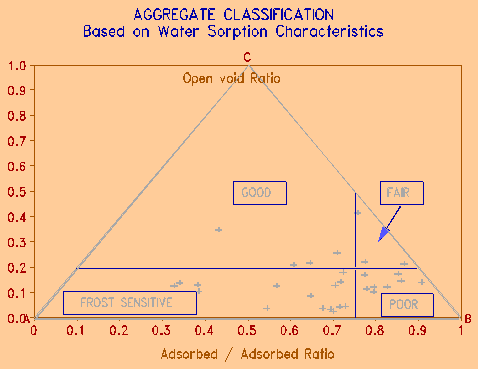
Figure 5 Tri-plot of sorption values related to freeze-thaw resistance. |
The effects of grain size, pore size, and water sorption in the samples studied can be summarized in a triangular diagram originally published by Dunn and Hudec (1972), and shown in a slightly revised form in Figure 5. It illustrates the 'critical saturation' effect - i.e., separates the rocks that tend to saturate to over 80% of total void space when immersed in water. It further classifies the rocks that critically saturate by either immersion (designated as FROST SENSITIVE), or by high humidity alone (designated as POOR). The latter tend to deteriorate by wetting and drying alone, as well as by freezing and thawing. The diagram shows the importance of water sorption on durability properties of rocks. Salt increases both water adsorption and critical saturation in porous materials, moving the position of the sample toward the POOR area of the diagram.
CHEMICAL TREATMENTS AND RESULTS
The selection of treatment chemicals for experimentation was governed by the following considerations: potential for beneficiating effect, non-toxic and environmentally acceptable, and economically viable. Amine group of chemicals was tried originally, and proved to be effective; however, they are noxious chemicals, and being organic, are probably degradable with time. Phosphate group was finally selected as the most promising - inorganic, inexpensive, and benign. Ammonium phosphate (NH4)2 HPO4, and various Na K, and Ca mono- di- and tri- phosphates were tested.
The chemical treatment was applied as follows, and in each case followed by five cycle freezing and thawing:
A. Pre-treatment of selected dry aggregates.
B. Exposing aggregate to de-icing salt, drying and then treating it with the chemical.
C. Casting of mortar and concrete samples with:
i. pre-treated aggregate
ii. with non-treated aggregate and then treating the concrete/mortar
D. Mixing of chemicals in varying proportions with the de-icing salt, and the mixture used as saturating medium for freeze-thaw tests. The purpose of this procedure was to determine if the normal de-icing salt can be made less aggressive and still retain its de-icing properties.
E. Influence of the following variables:
i. chemical concentration
ii. length of treatment
iii. leaching of treated aggregate by water.
Only limited results of these experiments are presented in this paper; a more thorough compendium of results will be presented when all the experimental work is completed.
Treatment of Aggregate with Na- and Ca- Phosphate:
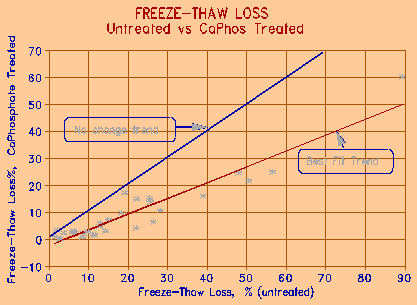
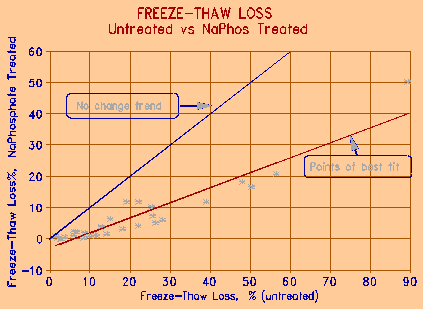
Figures 6 and 7 on the following page show the effect of treatment of dry aggregate by calcium and sodium phosphate, respectively. Calcium phosphate is relatively insoluble in water, and must be acidified to below pH 3 to be brought to solution. It is, however, slightly soluble in NaCl solution. Sodium phosphate (as most sodium compounds) is fully soluble. The treatment in both cases consisted of immersing dry aggregate sample in the phosphate solution for a period of 1 minute. The sample was then allowed to bench dry before being placed in a 3% NaCl solution for 24h saturation prior to freeze-thaw testing.
|
Figure 6. The effect of aggregate treatment with 5% Acidified calcium phosphate for 1 minute. |
|
Figure 7 The effect of treatment of aggregate with 5% Sodium Phosphate for 1 minute. |
The effectiveness of treatment is compared to freeze-thaw test results on the same untreated aggregates. As the figures show, there is a significant improvement in the resistance to freezing and thawing of the treated aggregates under what can be considered very severe conditions. It is interesting to note that the higher the freeze-thaw loss of the untreated sample, the more effective is the treatment in reducing the loss.
|
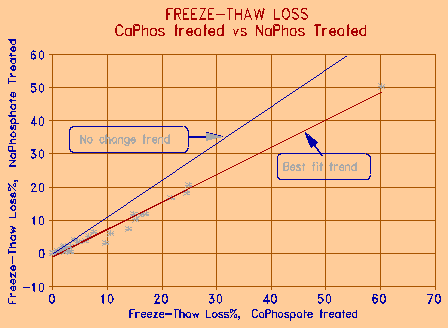
Figure 8 Comparison of the effectiveness of sodium and calcium phosphate treatments. |
Figure 8 compares the effectiveness of the two phosphate treatment chemicals. Calcium phosphate is, in general, about 20% less effective than sodium phosphate. The acidified calcium phosphate tends to precipitate in the surface environment of the aggregate particle, especially when the aggregate is, or contains carbonate. This may however, in the long run, make the calcium treatment more effective, since the precipitated phosphate will stay with the aggregate; sodium phosphate, because of its high solubility, may be subject to leaching. Although insoluble in water, calcium phosphate is slightly soluble in NaCl solution - the solution that causes most damage to concrete.
|
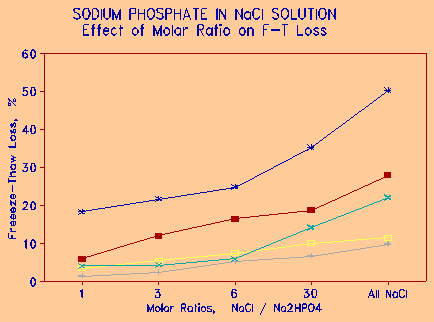
Figure 9 Reduction of freeze-thaw damage by admixtures of Na2HPO4 in NaCl. |
Figure 9 illustrates the effect of concentration of sodium phosphate in the freeze-thaw solution on the freeze-thaw loss. The concentrations are expressed as molar ratios - i.e. ratio of no. of molecular weights of NaCl to Na2HPO4. The molar ratios of 1, 3, 6, and 30 translate to phosphate concentrations in the salt mixture of 71, 49, 29, and 7.5 respectively. It is seen that even small amount of sodium phosphate in salt solution decreases the freeze-thaw loss significantly. Sodium or calcium phosphate could thus be used as a salt admixture, providing de-icing action, but reducing the deleterious effects associated with NaCl salt. Phosphate is a well known corrosion inhibitor; simple tests have shown that even small amounts of phosphate in the salt reduces the corrosion of metals. The downside of using phosphates, especially a soluble phosphate, is the potential eutrophication effect of rivers and lakes. More work needs to be done to investigate this.
|
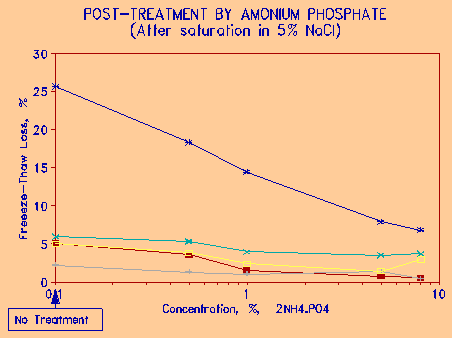
Figure 10 Treatment of NaCl-saturated aggregates with Ammonium Phosphate and its effect on Freezing and Thawing loss. |
Ammonium phosphate was also shown to be an effective treatment for aggregates. Because of ammonium, the salt is somewhat noxious in large concentrations, but probably acceptable in amounts that were found to be effective. Figure 10 shows the effect of treating previously salted aggregate with ammonium phosphate solutions of varying concentrations by five minute immersion. It is seen that the effectiveness of the treatment is a log function of concentration - i.e., very small concentrations have a significant effect, and increasing the concentration of the phosphate results in some, but not proportionate increase in benefit. Concentrations of 1 percent of this chemical would appear to be the optimum in terms of concentration and effect. Although not compared directly to other phosphates, ammonium phosphate would seem to be more effective in lower concentrations than either sodium or calcium phosphate.
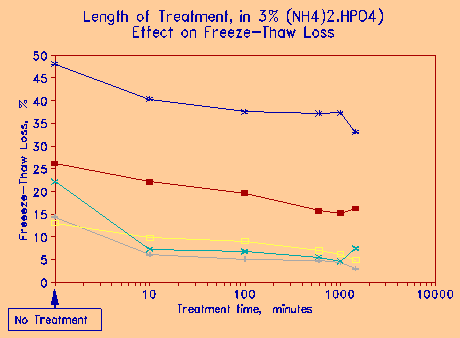
The effect length of exposure to the treatment chemical on freeze-thaw deterioration is considered next. Figure 11 gives the results for ammonium phosphate. Dry aggregate was exposed to a 3% solution for 1, 10, 100, 600, and 1000 minutes respectively. The relationships are shown to be log-normal, i.e., the longer the treatment, the more improvement there is; however, the amount of improvement is not significant after certain period of exposure. From practical point of view, the most effective exposure time is between 5 and 10 minutes.
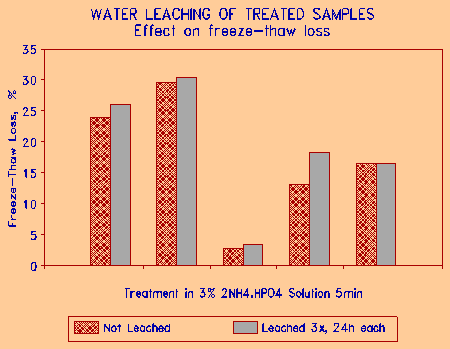
The question of effectiveness of treatment with time was addressed in the next set of experiments. Samples treated in 3% ammonium phosphate solution for 5 minutes were allowed to dry, and then leached in running water for 24h. After drying, the 24h leaching cycle was repeated two more times. The samples were then subjected to freezing and thawing. The results on five different aggregate samples are given in Fig. 12. It is seen that leaching did decrease the resistance to freeze-thaw degradation, but the amount of decrease was marginal. This suggests that this treatment chemical is effectively bound to the internal surfaces of the aggregate pores, and is able to withstand severe leaching. The laboratory leaching was probably more severe than may be encountered in service.
|
Figure 11 Effect of length of exposure to the treatment chemical on freeze-thaw durability. |
DISCUSSION AND CONCLUSIONS
The results presented above show that some chemicals, especially phosphates, are effective in controlling the destructive effect of de-icing salt during freezing and thawing. The effectiveness of phosphates is probably due to the ability of the phosphate anion to bind to and satisfy the charge of the Na+ ion. The phosphate anion thus reduces the clustering of polar water molecules around the Na+ ion, decreasing the osmotic pressure difference between the water in very small pores and the water in larger pores or the outside of the aggregate.
The treatment by surface-active chemicals applies not only to aggregate, but also to any porous building material. Surface treatment of mortar and concrete was equally successful in suppressing the freeze-thaw damage from de-icing salts. Some initial tests suggested that incorporating small amounts of some of the treatment chemicals in the mortar and concrete mixtures may also be an effective way in reducing the de-icing salt damage. More work is continuing along these lines.
The surface active chemicals have a potential in being used in three different ways:
|
Figure 12 Effect leaching of treated aggregate on the freeze-thaw durability. |
1. Topical or surface application to aggregate prior to its use in concrete.
2. Surface treatment of concrete or mortar.
3. Chemical admixture (in mixing water) during concrete batching.
It should also be mentioned that, not surprisingly, the phosphates were found mildly effective in reducing alkali reactivity of concrete and mortar under conditions 1 and 3 above. Alkali silica and silicate reaction takes place when free alkalis Na and K abound hardened concrete which contains reactive aggregate. The alkalis are derived from the cement, and from the de-icing salts. The phosphate, because of its affinity for any free cation, binds with the alkalis, and reduces the alkali reaction. This is an added incentive to further investigate the use of surface-active chemicals to improve the resistance of mortar and concrete.
ACKNOWLEDGMENT
The work described above was supported by an operating grant from National Science and Engineering Research Council of Canada (NSERC) to the senior author.
REFERENCES
Cady, P.D., Kline, D.E. and Blankenhorn, P.R., 1978, Deep impregnation of concrete bridge decks with linseed oil, Highway Research Record, pp.183-188
Dunn, J.R. and Hudec, P.P., 1972, Frost and sorption effects in argillaceous rocks, Highway Res. Rec., no. 393, pp.65-78
Hudec, P.P., 1989, Ionic control in deterioration of building materials, Water-Rock Interaction, Miles (ed), Proc. 6th Symp. on Water-Rock interaction, Balkema, Rotterdam, pp. 305-308
Hudec, P.P., 1989, Durability of rock as function of grain size, pore size, and rate of capillary absorption of water, Jour. Materials in Civil Eng., v.1., n.1, pp 3-9
Hudec, P.P., 1987, Deterioration of aggregates - the underlying causes, Amer. Conc. Inst. SP-100, v2, 1325-1342
Ministry of Transportation, Ontario, 1989, Method of test for freezing and thawing of coarse aggregate, Laboratory Testing Manual, LS-624,
Ministry of Transportation, Ontario, 1989, Procedure for the petrographic analysis of coarse aggregate, Laboratory Testing Manual, LS-609,
Ohama, Y., Sato, Y., and Nagao, H., 1987, Improvements in watertightness and resistance to chloride ion penetration of concrete by Silane impregnation, Proc. 4th Int. Conf. Durab. Build. Mat. & Comp., Singapore, Pergamon Press, v.1, pp.295-302
Verbeck, G. and Landgren, R., 1960, Influence of physical characteristics of aggregates on frost resistance of concrete, Proc. ASTM, v.60, pp.1063-1079