Chemical Treatments And Additives To Reduce Alkali Reactivity:
Successes and Failures
Peter P. Hudec
Synopsis: The deterioration of concrete due to alkali silica reactivity (ASR) is caused by a three-stage process: the dissolution of silica from susceptible aggregates, the formation of silica gel, and the expansion of the gel. Thus, stopping or reducing the process at any of the three stages should arrest ASR.
Certain chemicals that could potentially: a) stop or reduce the dissolution of silica, b) interfere with either the formation or character of the silica gel, and c) reduce the expansivity of the gel have been tried as additives in mortar, and as surface treatments of hardened mortar. The effectiveness of the chemicals was measured by rapid alkali reactivity (AR) testing method (expansion after exposure to 1N NaOH at 80oC).
The results suggest that the phosphate and nitrate anions, and calcium, magnesium, and lithium cations appear to have beneficial effects. Phosphate and nitrate reduces the solubility of silica. The effect of cations is not clear: they may replace the Na and K of the cement alkalies in the silica gel, making the gel less expansive. It is interesting that the anions of phosphate and nitrate also tend to reduce freeze-thaw damage due to de-icing salts.
The effects of these additives on the strength properties of the mortar have been examined. Increases in unconfined uniaxial strength and sonic velocity were observed with some additives at low concentrations, and decreases at higher (15% of mix water) concentrations.
The improvements in ASR resistance were not uniform across all aggregate types tested. In some cases, increased ASR was observed.
Hudec, Peter P., 1991, Chemical treatments and additives to reduce alkali reactivity: Successes and Failures; Amer. Conc. Inst. Supp. Paper, pp. 235-249.
INTRODUCTION
Alkali silica(te) reactive aggregates have one common characteristic: they have large active surface areas. The large surface areas are attributed to the fine grained texture of most of alkali reactive (AR) aggregates: As grain size decreases, the surface area per unit weight of mineral increases. In addition to being large, the surfaces are also active, because they consist of either strained or imperfect crystal lattices which attract charged ions to their surfaces and the lattice. The alkalies in the paste are attracted to these large active surfaces. Most of these surfaces are negatively charged, and attract positively charged Na, K, and Ca cations. The cations become adsorbed to the active surfaces and attract OH- anions out of pore water. The OH- ion promotes silica dissolution, which results in the formation of the silica gel (1). The removal of OH- out of solution when in contact with amorphous silica gel solid has been experimentally demonstrated (2). The alkalies at the silica(te) interface then become incorporated and concentrated in the silica gel. The concentrated alkalies set up osmotic potential which attracts water molecules, causing pressure and expansion (3).
The above scenario is one explanation of alkali reactivity. It explains why no expansion occurs in some concretes, even if silica gel forms - i.e., lack of excess alkalies to initiate osmotic cells. It also explains why accelerated alkali reaction occurs in the presence of de-icing salt, such as NaCl, which provides additional Na, but does not appreciably increase the pH of the solution - a requirement for dissolution of silica. Although the potential for gel formation is present in every concrete containing reactive aggregate, expansion takes place only when a certain level of alkalies are present. The alkalies are usually provided by the cement, or by an external source such as the de-icing salt.
Lessening the amount of gel formation in presence of alkalies, or reducing the amount of alkalies, or interfering with the alkalies in the presence of gel will likely reduce AR expansion. The research described herein focused on achieving one or the other conditions with certain cationic and anionic species thought to influence the surface physical chemistry of the aggregate. Previous research on freeze-thaw durability of concrete and aggregate (4) has shown that large, strongly electro-negative anions such as phosphate decrease the damage the de-icing salts (NaCl, CaCl2) cause to aggregate and concrete. On-going research has also shown that some cations, notably Li, also decrease the de-icing salt damage. Since much of the damage is thought to be due to osmotic swelling and not freezing and thawing, the same ions may also decrease osmotic expansion caused by cation-rich silica gel in ASR.
RESEARCH METHODOLOGY
The reactive aggregates used in the study were: Sudbury silicate reactive, Spratt silica reactive, Putnam silica reactive, and Kingston carbonate-reactive material. With the exception of Putnam, all aggregate was obtained from the Ministry of Transportation (Ontario) (MTO) stock piles in Toronto. Putnam aggregate was collected as chert nodules from gravels east of London, Ontario. The aggregates thus represent five diverse types of alkali reactive material. A non-reactive dolostone from Manitoulin Island, Ontario, was used as a control. The mortar mix design followed ASTM C227 specification, with water-cement ratio of 0.5. The pessimum proportion of Putnam Chert aggregate of 25% was used, the remainder being inert dolostone. Pessimum content was determined experimentally.
Details of Testing Method
The Oberholster and Davis (5) or the NBRI test uses mortar bars of the ASTM C227 specification, and 1N NaOH immersion at 80oC to accelerate the alkali silica reactivity. The test. This procedure was modified for use in this laboratory. The main modification involved the specimen size and the method of measuring, and is more fully described elsewhere (6). Briefly, rather than casting three mortar bars for each test, a single block of either concrete or mortar is cast. After 24-h set, the block is de-moulded and cured for 24h in 80oC water. Three cores of either 19mm or 25mm diameter are cut from the block; the remainder of the block is stored moist for additional testing, if required. The cores are kept moist at all times, and are squared and `dimpled' at ends to allow them to fit into the measuring equipment.
The equipment consists of a double linear variable differential transducer (LVDT). One LVDT is used to set the original length of the sample, and the second reads the changes in length during the experiment. The sample length is measured seven times and the 'cleaned' mean (to +/-1 standard deviation) is extracted. The means for the three cores per sample are likewise `cleaned'. The equipment is capable of measuring to 0.1 m sensitivity. The sensitive equipment, multiple measurements and statistical `cleaning' is necessitated by the small length of the sample -about 70mm. The final result is considered to be a very accurate measure of the sample's length.
The cores are placed in the hot 1N NaOH environment for 2 days, cooled to
room temperature to facilitate handling, and their length is measured. The cores
are then returned to the hot environment; the cycle is repeated for a minimum of
14 and maximum of 21 days. The number or cycles depends on the nature of
expansion observed.
Selection of Test Chemicals
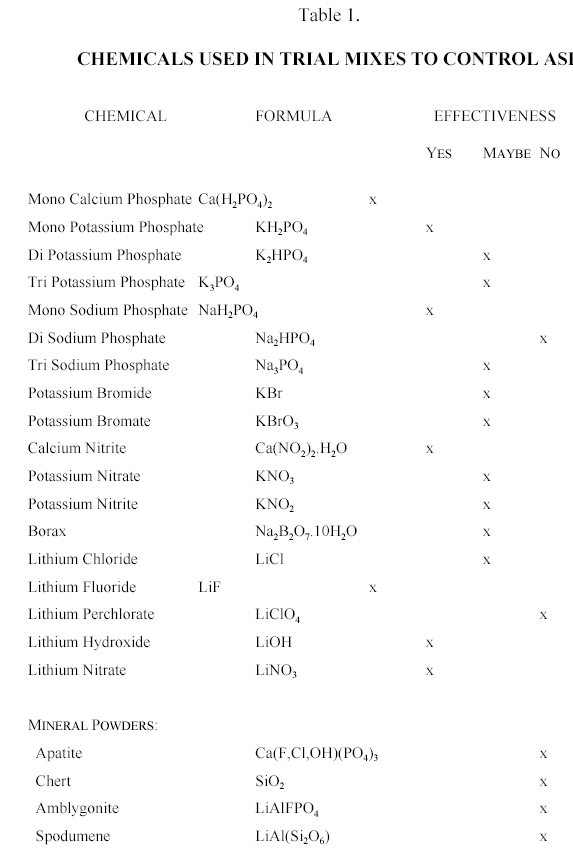
The selection of the chemicals that may reduce or eliminate AR was based on several criteria: their solubility, and potential for providing anions, cations, or both. In the case of anions, the potential for reducing silica solubility was considered as one of the main factors. Anionic species such as phosphates, borates, and nitrates were thought to be good candidates.
In the case of cations, the ability of the cation to compete with and replace the alkali cations (Na, K) in the silica gel was the main consideration. Lithium is already known to be an AR retardant, so it was a prime candidate. A number of Li-bearing compounds have been tried by this and other workers, and not all worked (7,8). It is also known that abundance of calcium, either as hydroxide or Ca2+ in the paste tends to retard AR, so some calcium salts were also tested. The problem with calcium (and magnesium) is that they tend to form compounds of low solubility. Calcium hydroxide initially promotes gel formation (9); however, it has been shown that concrete with excess of Ca2+ ion in the pore water does not expand as much as the one without, all other conditions being equal (10,11). Unfortunately, most Ca compounds are water insoluble, and difficult to maintain in concentrations that would counteract alkali ion concentrations. The chemicals were introduced as solutions in the mix water at various concentrations. Any unusual behaviour of the wet mix, and its setting was noted.
The third class of additives were minerals of phosphate and lithium. The minerals are inert, and would cause minimum of disruption of known paste properties, and are also environmentally benign. Powders of the minerals were added, usually as a 10% addition of cement weight.
The list of the chemicals used is given in Table 1.
EXPERIMENTAL RESULTS
The volume of experimental data collected is much too large to be presented in this format. Only the results that have a direct bearing on the chemistry of the alkali reactive process are presented.
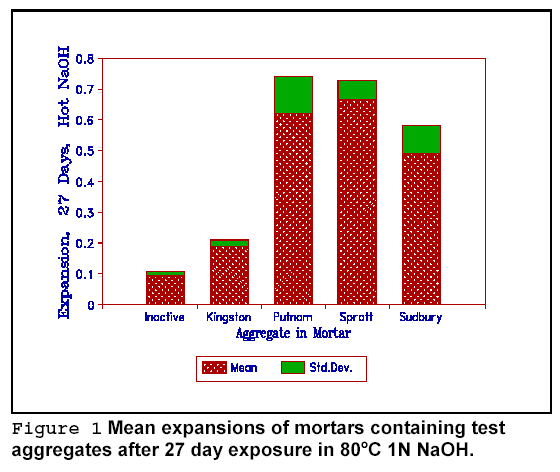
Figure 1 shows the mean expansion of the reactive aggregates tested in untreated mortar. Two points of interest should be noted. The rapid AR test as used here also expands carbonate-reactive aggregate slightly, as also reported by Grattan-Bellew (x). The variability in the results, shown by the standard deviation bar, is largely due to minor changes in the mix design during for each of the runs.
The Anion Treatment
There are several ways by which an alkali cation can be immobilized. One
method is to sorb the cation by an ion exchange resin. This has been shown to
work to a limited extent. Another way is to chemically precipitate it.
Unfortunately, Na and K ions form mostly soluble salts, and are difficult to
isolate. The third method is to ionically bind the alkalies to a `strong' anion
- one with large charge/radius ratio. The anion, in effect, competes for the
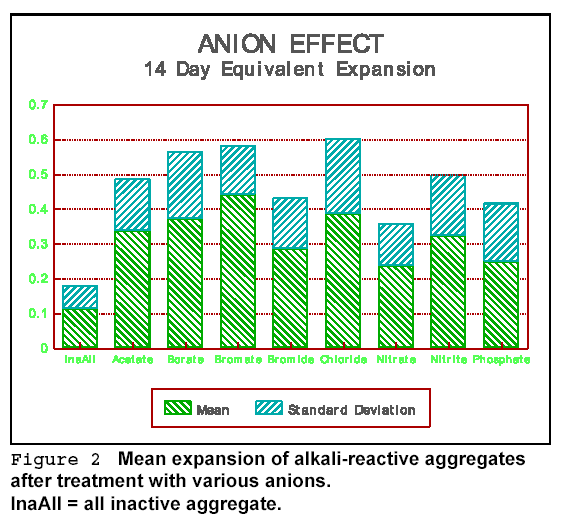
alkalies with the silica gel. The effect of various anions on alkali expansion
is shown in the bar graph of Figure 2. The figure represents mean values for all
the tests done on AR aggregates with various concentrations of anionic salts,
such as mono, di, and tri potassium phosphates, and various calcium and sodium
phosphates. As a result, there is considerable scatter, as shown by the standard
deviation bar. However, since all aggregates were subjected to the same
treatments, the means can be directly compared. Note that nitrate and phosphate
anions caused the highest reduction of AR expansion.
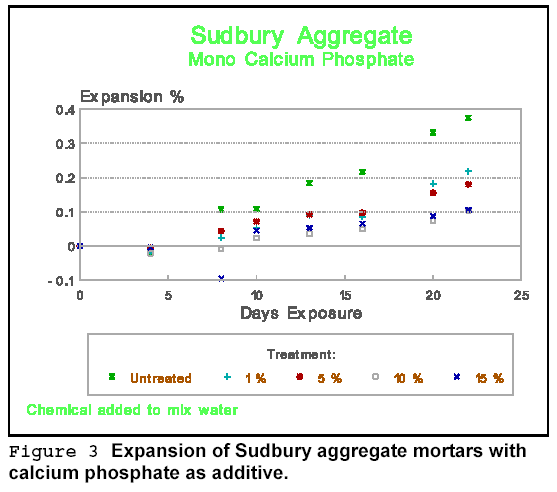
Consider phosphate. It solution it becomes strongly acidic, reacting with Na and K hydroxides. Figure 3 shows the expansion of Sudbury aggregate mortar with 0, 1, 5, 10, and 15% of monocalcium phosphate monohydrate in mix water. In general, as the phosphate concentration increases, the expansion decreases. Although phosphate appears to be effective, it is not equally so with all silica reactive aggregates. Calcium phosphate is relatively insoluble, and was added to the mix in an acidified (with phosphoric acid) form. Upon contact with the cement, the phosphate precipitated into fine calcium phosphate powder.
Figure 4 shows the effect of mono potassium phosphate on ASR expansion. The 15% concentration in mix water gave the best results; curiously, the 5% but not the 10% concentration also showed some improvement. This may be a real effect, or an experimental error. Using this salt adds more undesirable alkali to the mortar, and this may introduce complications. A similar trend was observed with monosodium phosphate as well. There may be some balance between the amount of alkalies and soluble phosphate that must be struck in order for the salt to be effective. It was also noted that if di- or tri- alkali salts of phosphate were used, an increase in ASR expansion rather than a reduction was observed. This is being investigated further.
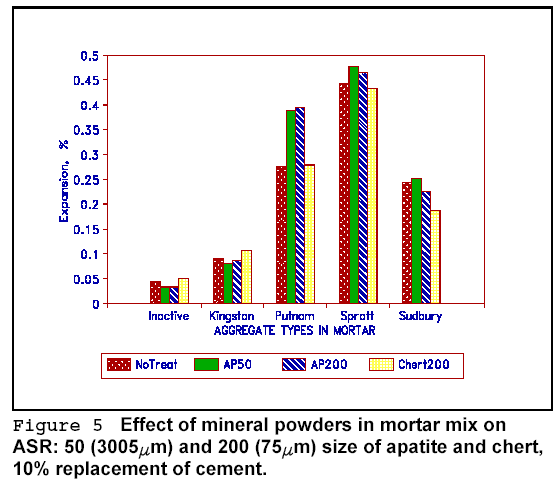
To test the effectiveness of inert calcium phosphate on ASR, mineral apatite was ground and added to the mix. The results are shown in Figure 5. The figure also gives the effect of powdered chert on ASR. The apatite increased the expansion in most cases. The ground chert (probably acting as a pozzolan) decreased the expansion slightly. Powdered lithium minerals amblygonite (LiAlFPO4) and spodumene (LiAl(Si2O3) ) were tested as partial replacement of cement. No reduction in expansion was observed.
Nitrates and phosphates reduce ASR. However, phosphates may be more economic to use if this treatment proves viable, because of greater abundance of mineral phosphate deposits.
Cation Treatment
The role of alkalies (Na and K) in ASR is two-fold: they promote dissolution of silica and the formation of silica gel by increasing the pH of the pore solution. The increase in pH comes through the precipitation of sulphoaluminates and consumption of water by hydration. The remaining free alkalies then become adsorbed by the silica gel. Because of higher alkali concentration in pore fluids within the gel, water flows into the gel and exerts pressure. Thus, in alkali reactive mortars, replacing the high osmotic potential cations with those of lower osmotic potential may arrest expansion and cracking. One way of accomplishing this is by providing an excess of low osmotic cations such as calcium, magnesium, or lithium, which, by the law of mass action, will replace the less desirable Na and K cations in the gel.
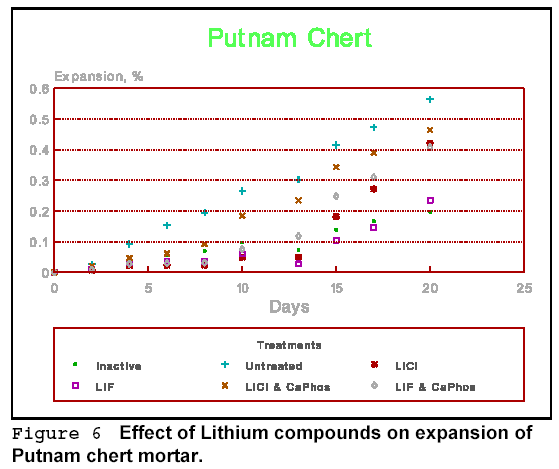
Figures 6 to 8 show the effect of some of the compounds, alone, or in combination with others on the expansion of Putnam chert mortar. All of the lithium combinations gave somewhat better results than an untreated mortar, but some were clearly better. Some lithium compounds, such as lithium acetate and lithium carbonate had no effect on AR expansion. The results with lithium carbonate are similar to those of Sakaguchi et al (7), but at odds with those of Ohama et al (8).
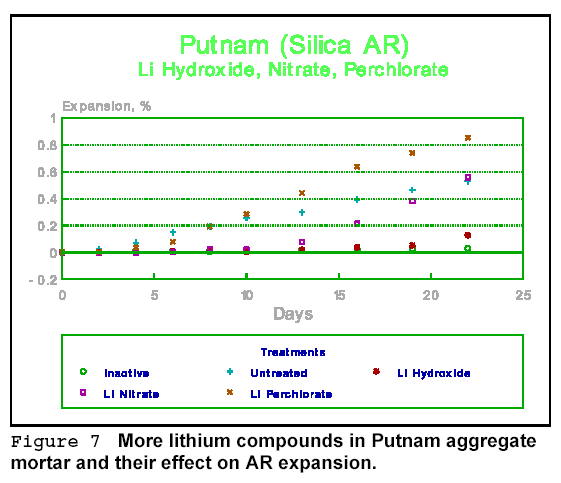
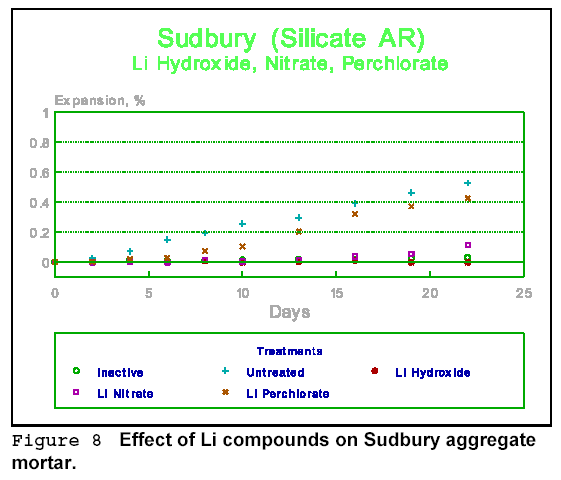
Figures 9 and 10 give the effect of more lithium compounds, this time on Sudbury aggregate mortar. The best treatment found so far, both among cations and anions, would appear to be lithium hydroxide. It works for most aggregate types. Lithium nitrate, on the other hand, works well in some, but not in others. This may indicate one of the problems with some chemical treatments - they tend to be aggregate specific.
There are other treatments being tried currently. One, which appears to have some promise, is the use of ion exchange materials in the concrete. Some of these materials are byproducts of other processes, others are mineral-based substances. Any material that has greater appetite for the cations than the silica gel should reduce expansion. Some encouraging preliminary results have been obtained using carbon-based surface-active products.
Effect of Chemical Treatments on Strength
Some preliminary measurements of the effect of chemical additives on strength
were made. The cores, after the rapid ASR test, were cut to 2:l ratio, and
subjected to unconfined uniaxial compressive strength test. Because the testing
was done on ASR-affected specimens, the results reflect both the effect of ASR
and the chemical on the ultimate strength of the mortar. However, it can be seen
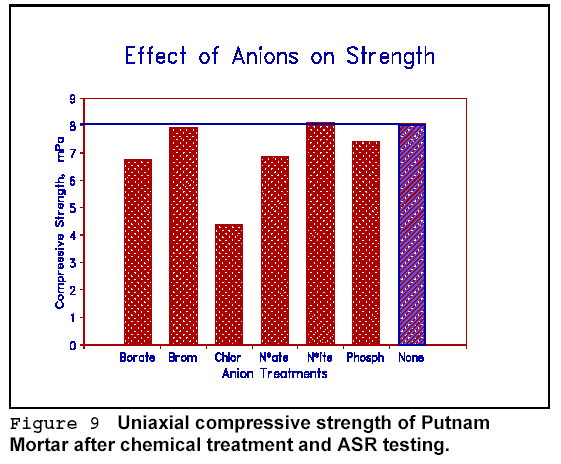
in Figure 9 that phosphate, bromide and nitrite have minimal effects on
compressive strength, while chloride adversely affects the strength.
Chloride-treated mortars also show maximum expansion under accelerated AR
testing, and this may explain their low strength. These results reflect the
average of all tests for a given anion, including several cationic types in the
average. The results given are those for Putnam chert mortars.
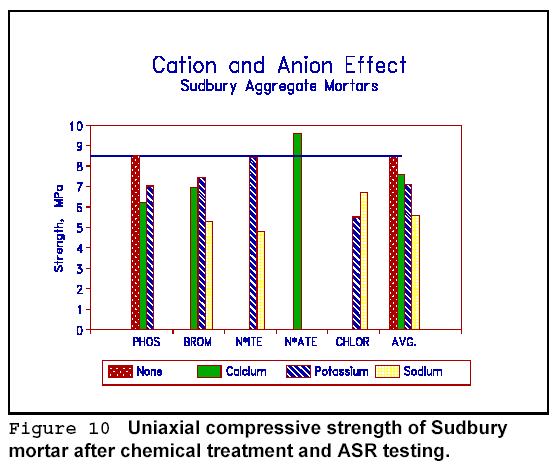
Figure 10 shows data for Sudbury aggregate mortars. Here, the results are
arranged to show the averages for each cationic and anionic species. Calcium
nitrite gives the best results. Again, chlorides as a group are poor performers.
There is a general, but not statistically strong correspondence between the
strength and the expansion due to AR.
No strength results were available for the lithium salts at the time of writing.
CONCLUSIONS
Fly ash, ground slag and silica fume are the current favourites for
controlling alkali reaction and expansion. Fly ash has proven to be somewhat
variable in its effectiveness, principally because it is waste product, whose
composition depends on the coal properties from which it is derived. As power
plants change fuel sources and methods of burning, the composition of the fly
ash and its ability to control AR also changes.
Phosphate, nitrate, nitrite, and bromide have shown some promise in reducing AAR. Lithium and calcium appear to be effective cations. A proper combination of soluble salts of effective anions and cations may prove to be quite effective - for instance, lithium phosphate. Relatively small amounts (5 to 10% by weight of mixwater) seem to control AR. Mono-cation salts are more effective than di- or tri-cation salts. Monovalent rather than divalent cations would seem to be more effective, since these can replace the alkali salts from the gel more easily.
These experiments are being conducted with the aim of developing a reliable, chemical control of AR-related expansion. The optimum dosages, effectiveness on a variety of AR aggregates, and the effect on other properties of the mortar need still to be investigated.
ACKNOWLEDGMENT
The research described above was supported by a grant from National Science and Engineering Council of Canada. The laboratory work was carried out by Mrs. E. Verespej, lab technician, and Mr. N. Banahene, graduate student.
REFERENCES:
1. Uhran, S., 1987 "Alkali Silica and Possolanic Reactions in Concrete. Part 1: Interpretation of Published Results and an Hypothesis Concerning the Mechanism"; Cement and Concrete Research, vol. 17, pp. 141-152.
2. Glasser, L.S. Dent, and Kataoka, N., 1981, "The Chemistry of
'Alkali-Aggregate' Reaction"; Cement and Concrete Research, vol. 11, pp. 1-9.
3. Hobbs, D.W., 1981, "The Alkali-silica Reaction - A Model for Predicting Expansion in Mortar; Mag. Concr. Res., v.33, 208-220.
4. Hudec, P.P., 1989, "Ionic Control in Deterioration of Building Materials"; Proc., 6th Inter. Symp. Water-Rock Interaction, A.A.Balkema, Rotterdam, pp.305-308.
5. Hudec, P.P., and Larbi, J.A., 1989, "A Study of Alkali-Aggregate Reaction in Concrete: Measurement and Prevention, Part I"; Cement and Concrete Research, vol. 19, pp.905-912.
6. Oberholster, R.E., and Davies, G., 1986 "An Accelerated Method for Testing the Potential Alkali Reactivity of Siliceous Aggregates"; Cement and Concrete Research, v. 16, pp. 181-189.
7. Sakaguchi, Y. et al, 1989, "The Inhibiting Effect of Lithium Compounds on Alkali-Silica Reaction"; Proc., 8th Inter. Conf. on Alkali-Aggregate Reaction, Soc. Mater. Science, Kyoto, Japan, pp. 229-234.
8. Ohama, Y, Demura, K., and Kakegawa, M., 1989, "Inhibiting Alkali-Aggregate Reaction with Chemical Admixtures" Proc., 8th Inter. Conf. on Alkali-Aggregate Reaction, Soc. Mater. Science, Kyoto, Japan, pp. 253-258.
9. Chatterji, S, Thaulow, N., Jensen, A.D. and Christenses, P., 1987, "Mechanism of Accelerating Effects of NaCl and Ca(OH)2 on Alkali-Silica Reaction"; Proc., 7th Inter. Conf. on Alkali-Aggregate Reactions, Ottawa, Noyes Publications, pp. 115-119.
10. Xu, Huayong, 1987, "On the Alkali Content of Cement in AAR"; Proc., 7th Inter. Conf. on Alkali-Aggregate Reactions, Ottawa, Noyes Publications, pp. 451-455.
11. Powers, T.C., and Steinour, H.H., 1955, "An Interpretation of Some Published Researches on the Alkali-Aggregate Reaction, Part 2"; Jour. Amer. Conc. Inst., v.51, pp.785-812.