Research Intersts
Central to understanding the chemistry of cells is elucidating the chemistry of important biomolecules and their interactions. A class of biomolecules of particular interest is enzymes; molecules that catalyse the broad range of reactions essential for life. In addition to the fundamental knowledge to be learned, this interest is due in part to the medical and industrial benefits to be obtained. For example, the aim of therapeutic drugs is often to enhance or inhibit the function of a specific enzyme. Computational chemistry uses computers to model the chemistry and reactions of chemical systems.
Such approaches are used, for example, to study problems that may be too difficult to study experimentally or to provide insight into observed phenomena. Our research group uses the methods of computational and theoretical chemistry to investigate the chemistry and reactions of various biomolecules, in particular biochemical catalysts. Brief overviews of some various areas studied by our group are described here.
A computational insight into the Sulfilimine bond formation in Collagen-IV
Anupom Roy
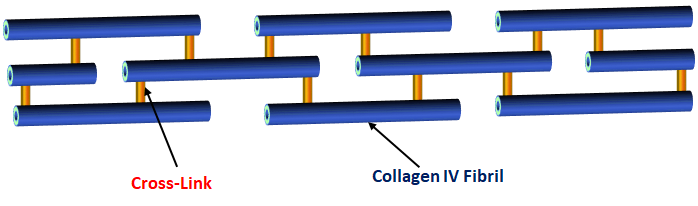 Collagen IV is composed of six highly homologous polypeptides. Usually, they are referred as α1(IV) to α6(IV). The α-chain can be classified into three domains - an amino-terminal, a triple-helical, and a carboxy-terminal domain. Furthermore, these three chains combine into a trimer which is a protomer that then aggregating end to end to form a hexamer. The protomers are covalently cross-linked which gives the structural integrity of collagen IV network.
Collagen IV is composed of six highly homologous polypeptides. Usually, they are referred as α1(IV) to α6(IV). The α-chain can be classified into three domains - an amino-terminal, a triple-helical, and a carboxy-terminal domain. Furthermore, these three chains combine into a trimer which is a protomer that then aggregating end to end to form a hexamer. The protomers are covalently cross-linked which gives the structural integrity of collagen IV network.
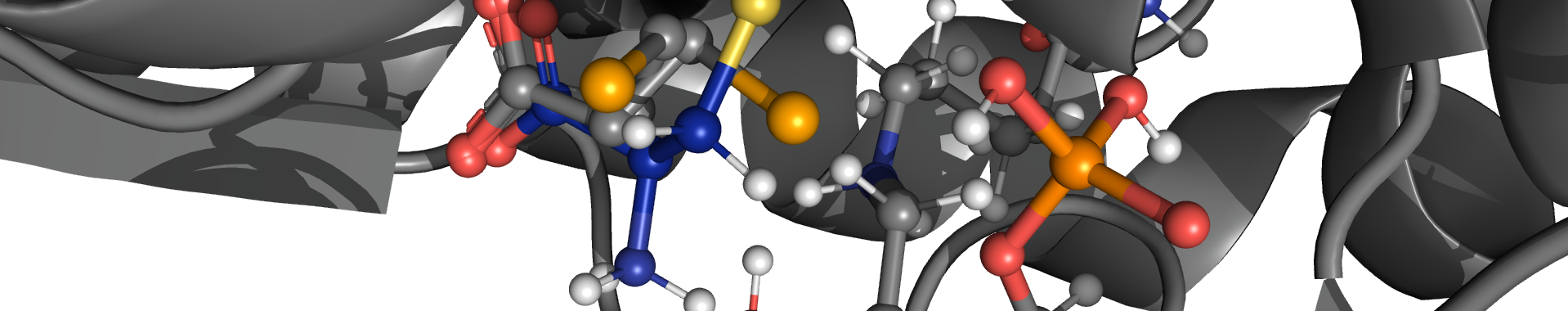
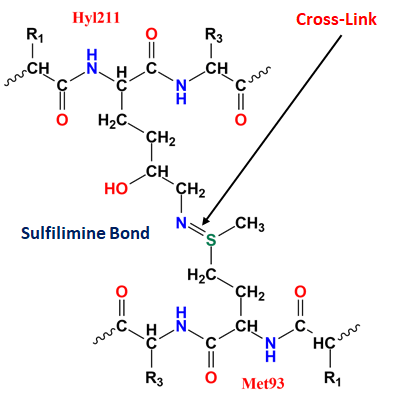 It is known that collagen IV undergoes several post-translational modifications (e.g., hydroxylation of lysine's). The α1, α2, and α1 collagen IV are found to be covalently cross-linked between hydroxylysine (Hyl211) and Methionine (Met93) residues. The formation of cross-link between those chains require post-translational modification.
It is known that collagen IV undergoes several post-translational modifications (e.g., hydroxylation of lysine's). The α1, α2, and α1 collagen IV are found to be covalently cross-linked between hydroxylysine (Hyl211) and Methionine (Met93) residues. The formation of cross-link between those chains require post-translational modification.
On the other hand, the cross-links of α3, α4, and α5 collagen IV are connected between Lys211 instead of Hyl211 and Met93 which also indicates that the post-translational modification is not essential for the formation of these cross-links. Therefore, it has already been reported that the cross-link can be formed with or without post-translational modification between lysine or hydroxylysine and methionine residues. This covalent crosslink is called sulfilimine (S=N) crosslink which is rare in biochemistry. This sulfilimine bond was discovered in 2009 and so far only being known to occur naturally in collagen IV.
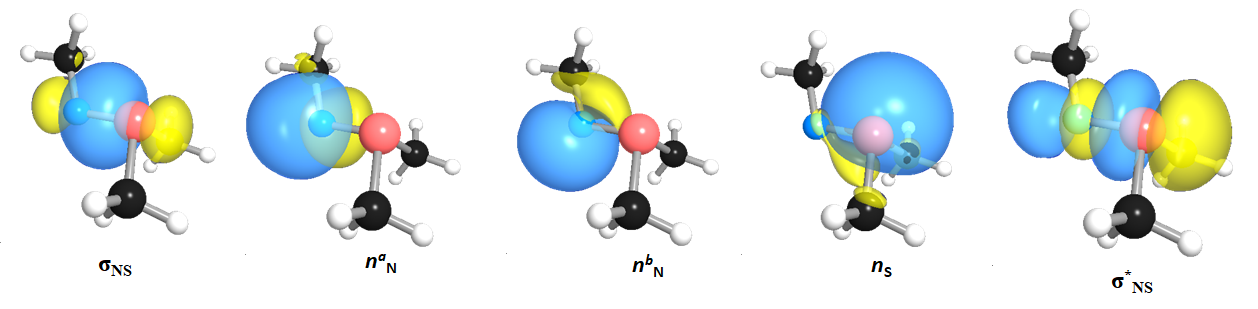
Computational Insights into Sulfur-Containing Compounds in Chemical Systems
Sahar Nikoo
Sulfur-containing molecules have been recognized as one of the versatile and significant molecular construction with a broad spectrum of potential, diversity, and application from biology, and enzymatic processes to organic synthesis and pharmaceutical industry. They can undergo a diverse variety of post-translational modifications from disulfide bond (S--S) formation, reaction with N-containing sources to form a sulfur-nitrogen bond (S--N) and metal-bonding within the biological environment to form a dozen of the functional group.
My doctoral work includes some sulfur-related projects in which the functionality, reactivity, and mechanism of the varied sulfur species in different environments has been examined computationally.
Multistate Reactivities of Non-Heme Enzymes
Dr. Abayomi Faponle
 We have broader interest in computational investigations of enzymatic reactions and catalytic mechanisms. We have expertise in multistate reactivities of nonheme and transition-metal containing nonheme enzymes that have relevance in drug metabolism, biotechnology, biofuel, health and diseases pathogenesis etc.
We have broader interest in computational investigations of enzymatic reactions and catalytic mechanisms. We have expertise in multistate reactivities of nonheme and transition-metal containing nonheme enzymes that have relevance in drug metabolism, biotechnology, biofuel, health and diseases pathogenesis etc.
Currently, we are investigating the mechanisms by which these enzymes use active metal centres to transfer sulfur into biomolecules of physiological significance.