 2
2To truly understand CT scanning, you must first learn about X-rays and how they are produced. X-rays are a form of light with a wavelength in the range of 0.1 nanometres to 10 nanometres. This extremely small wavelength indicates that the X-rays have a much higher energy than visible light. X-rays can be dangerous in high dosages; however the amount received during a CT scan is minimal and safe. In fact the radiation dose from this procedure is between 0.2 to 2.0 rads of radiation.
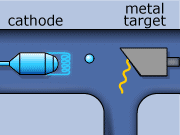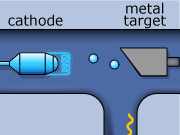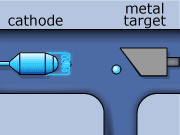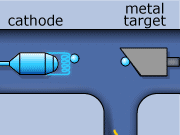
The X-ray production process begins by creating electrons with a metallic cathode (filament). In an evacuated space, electrons are boiled away from a hot filament, which is heated by an external current. These electrons are then accelerated though a large potential difference, and collide with heavy atoms in the metal anode. Most of the electrons’ energy is expended heating up the metal anode, while the rest is emitted in the form of photons in the X-ray range of frequency. Generally, the metal used as the anode has a high melting point, and is an excellent thermal conductor. The anode must have these properties if it is to withstand electron bombardment, and avoid over heating (i.e. melting). Tungsten incorporates these desirable characteristics, and is typically the material used for the anode construction.
X-rays for CT scans are made in two ways. One way involves an electron slowing down as it travels around an atom. The energy that is lost when the electron loses its momentum forms an X-ray. This is called “bremsstrahlung”, from the German word for ‘braking radiation’. The bremsstrahlung x-rays wavelength can vary over the x-ray spectrum.
The second method of X-ray production occurs when an inner shell electron is ejected out from an incoming electron from an electron and an outer shell electron down to replace a lowest shell electron. When this happens, an x-ray is formed. This lowest shell electron is ejected by the collision with the incoming electron. This method is called “K-shell” emission. K-shell emission produces X-rays at one wavelength only.
Check Your Understanding
What does 'bremsstrahlung' mean in English?
A. Reverse radation
B. Forward radation
C. Breaking radation
Which way of producing X-rays deals with an electron being knocked out of its orbit?
A. K-Shell Emission
B. Bremsstrahlung radiation
C. T-shell Emission
Which one of these facts is not a reason that Tungsten is used in electron tubes?
A. Tungstem will not melt during the x-ray production process
B. Tungstem is a heavy atom
C. Tungstem is cheap to buy