If a gas has a constant mass and is held at a constant pressure then the volume divided by the temperature is a constant value.
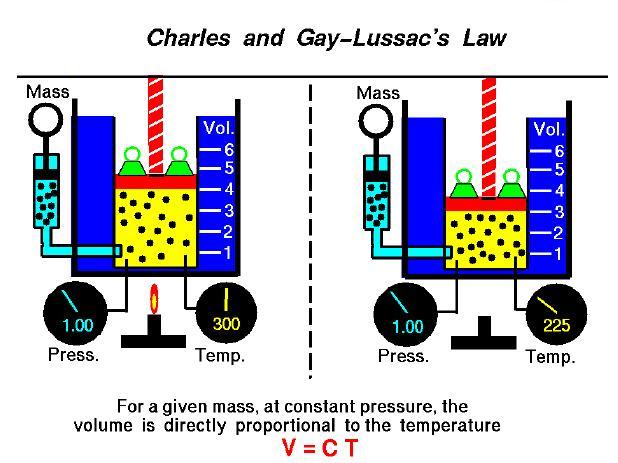
This law says that if I am holding a gas at a constant pressure and a constant mass and I increase the temperature then the volume of the gas will also increase. This makes sense if the particle model is used, since by increasing the temperature I am really just putting more energy into the system. This gives more energy to each of the particles inside the gas causing them to move more. As they move more and more the volume of the gas begins to increase.