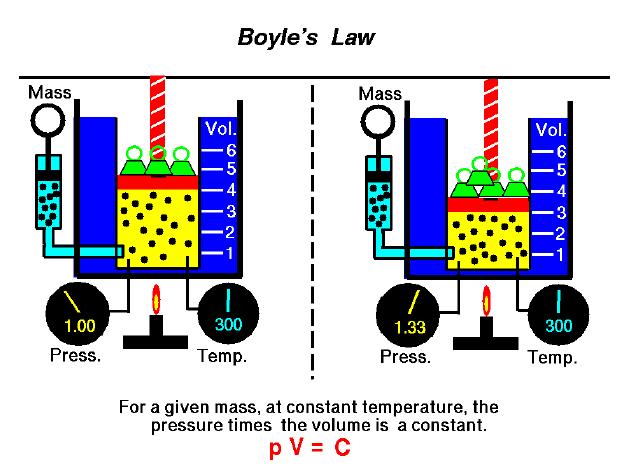
So this law says that if I am holding a gas at a constant temperature and a constant mass, then by increase the pressure of the gas, the volume of the gas will decrease and vise versa. This makes sense qualitatively because pressure is related to force. So if I push down on something "flexible" like a gas I will squish it, as a result it will occupy less volume.