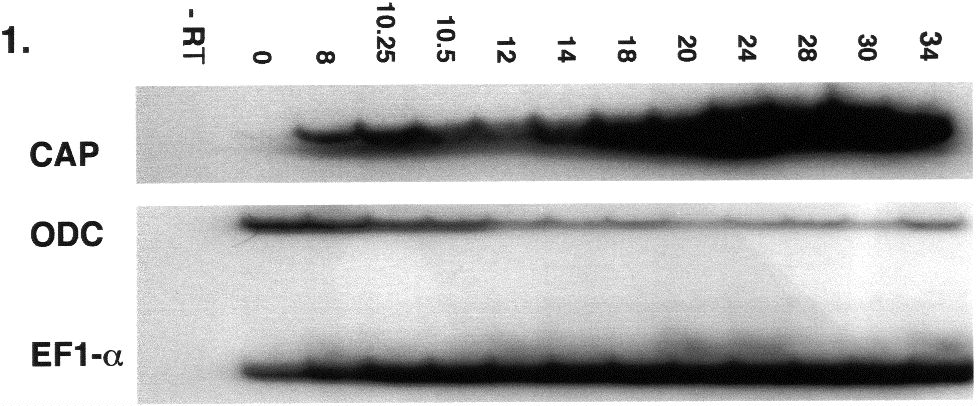
CAP1 expression is developmentally regulated in Xenopus.
F. KhosrowShahian, A.V. Hubberstey, M.J. Crawford*
Department of Biological Sciences, University of Windsor, 401 Sunset, Windsor, Ontario, N9B 3P4 CANADA
Received 7 September 2001; received in revised form 21 January 2002; accepted 23 January 2002
Abstract
We have cloned and characterized a Xenopus member of the CAP (cyclase associated protein) gene family. xCAP1 is expressed as a maternal transcript, but is up-regulated prior to gastrulation and subsequently localizes to head mesenchyme, lens, otic vesicle, and trunk mesoderm including the pronephros. At different stages, the gene also appears to differentiate surface from deep (sensorial) ectoderm. As in Drosophila, Xenopus CAP1 is expressed in the developing eye, specifically in the differentiating lens. However in distinction to Drosophila, Xenopus CAP1 does not express in periodically arrayed neural bands.
Keywords: lens, CAP1, cyclase associated protein, Xenopus laevis, branchial arch, ocular mesenchyme, sensorial ectoderm, pronephros, act up
1. Results and Discussion
The genes that encode cyclase-associated proteins (CAP) are conserved across organisms as divergent as plants, yeast, worms, flies, and mammals . CAPs are monomeric actin sequestering proteins that are thought to play a pivotal integrative role in linking cytoskeletal modifications with signal transduction pathways. Recently, a CAP homolog, act up, was shown to be necessary for normal oocyte polarity and imaginal eye furrow formation and differentiation in Drosophila .
We have isolated a Xenopus clone, xCAP1, which possess the entire conceptual open reading frame, and which would encode a protein with 80%, 79%, 79%, 54% amino acid identity to human, mouse, rat, and Drosphila CAP1 respectively. RT-PCR reveals that xCAP1 is detectable at low levels as a maternal transcript, but subsequently expresses at higher levels during blastula and later stages (Fig. 1). Whole-mount riboprobe in situ hybridization reveals the progressive restriction of xCAP to the animal pole with a presumptive dorsal bias (Fig. 2A, B). This bias, though hard to detect in early stages, is absent in sense controls, and is not seen when we probe with other faint maternally expressed probes such as Pitx1 and Pitx3. Dorsal restriction ensues (Fig. 2C, D), followed by expression in developing head (Fig. 2E,F,G,H). Ectoderm layers express in a differentially dynamic manner, with expression eventually restricting to the sensorial (deep) ectoderm (Fig.3A-D). xCAP1 appears to differentiate between surface and sensorial ectoderm earlier than previously recognized (Fig. 3A,D), and the period during which both layers express correlates well with the period when sensorial ectoderm cells migrate up into the surface ectoderm to give rise to ciliated cells through to late neurulation (Fig. 3B,C). During gastrulation and early neurulation
(Fig. 2C,D) xCAP1 is expressed in antero-dorsal mesoderm, and later in branchial arch and optic mesenchyme, lens vesicle, otic vesicle, and lateral mesoderm, but not endoderm (Fig. 2 E-H, Fig. 3). By stage 37, xCAP1 is expressed in the pronephros, rhombencephalon, and persists in the branchial arches, periocular mesenchyme, and lens through to late organogenesis. Sense controls were consistently clear of staining.
In Drosophila, CAP-modulated actin polymerization plays a fundamental role in eye differentiation (Benlali et al., 2000). Vertebrate eye development is also a multi-step process that requires specific inductive signals, morphogenetic movements, and dramatic cytoskeletal rearrangements . When lens ectoderm invaginates to form lens vesicles, the posterior lens epithelium cells lose their nuclei and elongate to produce primary lens fibers which then synthesize lens-specific proteins such as the crystallins. The xCAP —expressing anterior lens epithelial cells are fated to proliferate in the equatorial region of the lens and give rise to secondary lens fiber cells which eventually elongate and form lamellae surrounding the embryonic nucleus. The role of xCAP1 in this differentiation is currently being investigated.
2. Materials and Methods
2.1 Cloning. A partial fragment of xCAP1 was obtained using degenerate primers. The fragment was then subcloned and used to screen a Xenopus head and heart cDNA library (stages 28-35) which was constructed in commercially prepared vector (Stratagene). Dye terminator and dye primer chemistries were employed to bi-directionally cycle-sequence the largest obtained clone (2212 bp) which encompassed a complete open reading frame encoding a conceptual protein similar to CAP1 found in other species (xCAP1 accession #AF411959). Amino acids sequences were compared using the Clustal method.
2.2 Embryos and in situ hybridization. Embryos were fertilized, dejellied in 2% cysteine, cultured and staged as previously described . Wholemount in situ hybridizations were performed according to Harland (1991). Digoxygenein labeled sense and antisense riboprobes for CAP were generated from full-length linearized template. Dorsoventral dispositions of early cleavage stage blastomeres were identified and followed using regular furrow and colour determinants according Sive et al., (2000) and in situ hybridizations were thrice repeated in embryos derived from different egg clutches, and using different batches of riboprobe.
2.3 RT-PCR Total RNA from ten pooled embryos of each developmental stage was passed over oligo dT-polystyrene beads (Sigma DMN-10). mRNA equivalent to one embryo was withdrawn and reverse transcribed in the presence on RNasin (Promega) using reverse transcriptase (Omniscript, Qiagen). One fifth volume of this reaction was employed as template for amplification. PCR conditions were determined empirically to establish the linear range of amplification for xCAP1 using a thermo-stable polymerase in 10mM Tris (pH 9.0), 50mM KCl, 0.1% Triton X-100, 3 mM MgCl2, 0.2 mM dNTPs, 0.1mM [32P]dCTP, and 1 ug of each primer (CAP1 — CCACATCCTCAGAGATGAA and GGCTCTATACCCTTTATTAC; EF1-a — CAGATTGGTGCTGGATATG and ACTGCCTTGATGACTCCTA; ODC — GTCAATGATGGAGTGTATG and TCCATTCCGCTCTCCTGA). Following denaturation (3 minutes at 94oC), ODC and EF1-a were cycled 29x(94oC for 45oC seconds; 57oC, and 74oC for 45 seconds each ). xCAP1 assays were denatured (94oC for two minutes) and cycled 23x(94oC then 55oC for 45 seconds each; 72oC for 30 seconds). One tenth of each reaction was run out on 4% polyacrylamide in 0.5 x TBE, and then monitored by autoradiography.
Acknowledgements
Support for this work was provided by the Natural Sciences and Engineering Research Council of Canada in the form of grants to M.C.(203549) and A.H (203096). F.KS. was supported by Ontario Graduate Scholarship Awards.
References Cited

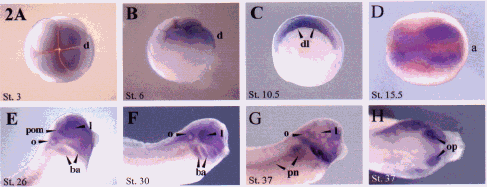
Fig. 2. xCAP1 expression during
embryogenesis. Maternal xCAP1 transcript becomes localized to the
animal pole during the early stages of cleavage (fig. A), and progressively
concentrates on the presumptive dorsal (d) side (figs. A, B). As the ectoderm
thickens, surface ectoderm down-regulates xCAP1 at the extreme animal
pole, while expression ensues in sensorial ectoderm, marginal zone and
dorsal mesoderm during gastrulation, so that cells immediately above and
passing through the dorsal lip (dl) express (fig. C). Late in gastrulation,
presumptive neurectoderm is devoid of xCAP1 expression anteriorly
(not shown), but by mid neurulation, the gene expresses in neurectoderm
up until the neural folds have sutured. Both layers of dorsal ectoderm
express during neurulation, as well as dorsal mesoderm and neurectoderm.
Dorsal expression extends in a comparable pattern from the yolk plug to
the anterior end (a), but is absent from endoderm from neural plate (stage
12) through to neural tube suturing (in this dorsal view of a stage 15.5
embryo)(fig. D). During elaboration of the head, xCAP1 is expressed
in the branchial arches (ba), otic vesicle (o), lens (l), and peri-optic
mesenchyme (pom) (figs. E, F). Branchial arch expression is predominantly
mesenchymal. By stages 36 to 37, olfactory placodes (op) and pronephric
structures (pn) express transcript (figs. G, H).
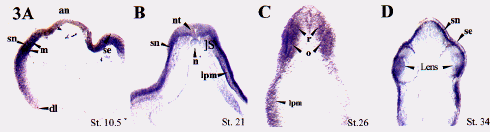
Fig 3. xCAP1 expression revealed in section. Animal pole (an) and surface ectoderm (se) do not express xCAP1 during gastrulation, though both mesoderm (m) and sensorial ectoderm (sn) do (fig. A). Cells passing through the dorsal lip (dl) appear to temporarily down-regulate xCAP1 indicating that expression patterns of this transcript can be rapidly altered. By late neurulation (fig B), both layers of ectoderm express transcript, but expression in surface ectoderm begins to diminish, while sensorial ectoderm (sn) and, to a lesser extent) lateral plate mesoderm (lpm) continue to express. Neither notochord (n) nor somites (s) express xCAP1, however a low level of expression can be seen in the lateral ventral aspect of the neural tube (nt). During tail bud stages (fig.C), transcript is detectable in the ventral rhombencephalon (r), the otic vesicle (o), and the lateral plate mesoderm (lpm), and expression in surface ectoderm has down-regulated. When eye begins to form, xCAP1 expression is detectable in the lens, particularly at the margins (fig.D). By this stage xCAP1 expression is entirely lacking in the surface ectoderm.